Abstract
Purpose
We used a Laser speckle flowgraphy (LSFG)-micro system to examine the relationship between ocular blood flow and retinal vascular endothelial growth factor (VEGF) at retinopathy onset in oxygen-induced ischemic retinopathy (OIR) model rats.
Methods
Sixteen 50/10 OIR rats were compared with 17 control rats reared in room air. In postnatal day 14 (P14) and P18 rats, we measured and analyzed the left eye’s mean blur rate (MBR) by setting a rubber band on the optic nerve head center, using the LSFG-Micro. At P18, the rats were sacrificed and their left-eye retinas were fixed, flat-mounted and stained with adenosine diphosphatase (ADPase). The right-eye retinas were homogenized; the lysate was centrifuged for an enzyme-linked immunosorbent assay (ELISA). The avascular area was measured as the percentage (%AVA) of the total retinal area. Retinal VEGF was measured by an ELISA.
Results
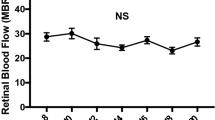
The examination’s reproducibility was good. Our multivariate linear mixed model analysis revealed significantly high MBRs in the OIR rats (p = 0.0017). In the P18 OIR rats, significant correlations were seen between the MBR and %AVA (r = 0.80, p = 0.0002) and between the MBR and VEGF (r = 0.76, p = 0.0006).
Conclusions
The LSFG-Micro provided reproducible blood flow measurements in neonatal rats. Because of the vitreous blood vessels, measurement of only the retinal vessels was not possible. However, the MBR was higher in the OIR rats than in the control rats, and the MBR and %AVA were correlated, as were the MBR and retinal VEGF. The MBR may thus serve as an indicator of OIR severity.





Similar content being viewed by others
References
Chen J, Smith LE (2007) Retinopathy of prematurity. Angiogenesis 10:133–140. doi:10.1007/s10456-007-9066-0
Holland DR, Saunders RA, Kagemann LE, Bluestein EC, Hutchinson AK, Corson DW, Harris A (1999) Color doppler imaging of the central retinal artery in premature infants undergoing examination for retinopathy of prematurity. J AAPOS 3:194–198
Niwald A, Gralek M (2006) Evaluation of blood flow in the ophthalmic artery and central retinal artery in children with retinopathy of prematurity. Klin Ocz 108:32–35
Neely D, Harris A, Hynes E, McNulty L, McCranor L, Siesky B, Plager D, Sprunger D, Roberts G (2009) Longitudinal assessment of plus disease in retinopathy of prematurity using color Doppler imaging. J AAPOS 13:509–511. doi:10.1016/j.jaapos.2009.08.012
Hartenstein S, Muller B, Metze B, Czernik C, Buhrer C (2015) Blood flow assessed by color Doppler imaging in retinopathy of prematurity. J Perinatol 35:745–747. doi:10.1038/jp.2015.45
Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H (1994) Noncontact, two-dimensional measurement of retinal microcirculation using laser speckle phenomenon. Invest Ophthalmol Vis Sci 35:3825–3834
Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H (1995) Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res 60:373–383
Tamaki Y, Araie M, Tomita K, Nagahara M, Tomidokoro A, Fujii H (1997) Real-time measurement of human optic nerve head and choroid circulation, using the laser speckle phenomenon. Jpn J Ophthalmol 41:49–54
Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S (2010) Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol 88:723–729. doi:10.1111/j.1755-3768.2009.01586.x
Isono H, Kishi S, Kimura Y, Hagiwara N, Konishi N, Fujii H (2003) Observation of choroidal circulation using index of erythrocytic velocity. Arch Ophthalmol 121:225–231
Fujii H (1994) Visualisation of retinal blood flow by laser speckle flowgraphy. Med Biol Eng Comput 32:302–304
Aizawa N, Nitta F, Kunikata H, Sugiyama T, Ikeda T, Araie M, Nakazawa T (2014) Laser speckle and hydrogen gas clearance measurements of optic nerve circulation in albino and pigmented rabbits with or without optic disc atrophy. Invest Ophthalmol Vis Sci 55:7991–7996. doi:10.1167/iovs.14-15373
Aizawa N, Yokoyama Y, Chiba N, Omodaka K, Yasuda M, Otomo T, Nakamura M, Fuse N, Nakazawa T (2011) Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin Ophthalmol 5:1171–1176. doi:10.2147/OPTH.S22093
Shiga Y, Omodaka K, Kunikata H, Ryu M, Yokoyama Y, Tsuda S, Asano T, Maekawa S, Maruyama K, Nakazawa T (2013) Waveform analysis of ocular blood flow and the early detection of normal tension glaucoma. Invest Ophthalmol Vis Sci 54:7699–7706. doi:10.1167/iovs.13-12930
Maeda K, Ishikawa F, Ohguro H (2009) Ocular blood flow levels and visual prognosis in a patient with nonischemic type central retinal vein occlusion. Clin Ophthalmol 3:489–491
Matsumoto T, Itokawa T, Shiba T, Katayama Y, Arimura T, Mizukaki N, Yoda H, Hori Y (2015) Reproducibility of neonate ocular circulation measurements using laser speckle flowgraphy. Biomed Res Int 2015:693056. doi:10.1155/2015/693056
Wang L, Cull GA, Piper C, Burgoyne CF, Fortune B (2012) Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci 53:8303–8309. doi:10.1167/iovs.12-10911
Cull G, Burgoyne CF, Fortune B, Wang L (2013) Longitudinal hemodynamic changes within the optic nerve head in experimental glaucoma. Invest Ophthalmol Vis Sci 54:4271–4277. doi:10.1167/iovs.13-12013
Wada Y, Higashide T, Nagata A, Sugiyama K (2016) Longitudinal changes in optic nerve head blood flow in normal rats evaluated by laser speckle flowgraphy. Invest Ophthalmol Vis Sci 57:5568–5575. doi:10.1167/iovs.16-19945
Cairns JE (1959) Normal development of the hyaloid and retinal vessels in the rat. Br J Ophthalmol 43:385–393
Liu K, Akula JD, Falk C, Hansen RM, Fulton AB (2006) The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 47:2639–2647. doi:10.1167/iovs.06-0016
Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL (2008) Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci 49:3107–3114. doi:10.1167/iovs.08-1780
Davitt BV, Wallace DK (2009) Plus disease. Surv Ophthalmol 54:663–670. doi:10.1016/j.survophthal.2009.02.021
Hartnett ME (2010) The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol 120:25–39. doi:10.1007/s10633-009-9181-x
Matsumoto T, Itokawa T, Shiba T, Katayama Y, Arimura T, Hine K, Mizukaki N, Yoda H, Hori Y (2016) Ocular blood flow values measured by laser speckle flowgraphy correlate with the postmenstrual age of normal neonates. Graefes Arch Clin Exp Ophthalmol 254:1631–1636. doi:10.1007/s00417-016-3362-6
Yamada Y, Suzuma K, Matsumoto M, Tsuiki E, Fujikawa A, Harada T, Kitaoka T (2015) Retinal blood flow correlates to aqueous vascular endothelial growth factor in central retinal vein occlusion. Retina. doi:10.1097/IAE.0000000000000595
Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP (2003) VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci 44:2155–2162
Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D'Amore PA, Shima DT, Adamis AP (2003) VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med 198:483–489. doi:10.1084/jem.20022027
Rakoczy PE, Brankov M, Fonceca A, Zaknich T, Rae BC, Lai CM (2003) Enhanced recombinant adeno-associated virus-mediated vascular endothelial growth factor expression in the adult mouse retina: a potential model for diabetic retinopathy. Diabetes 52:857–863
Edelman JL, Lutz D, Castro MR (2005) Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res 80:249–258. doi:10.1016/j.exer.2004.09.013
Kaur C, Sivakumar V, Foulds WS (2006) Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci 47:1126–1141. doi:10.1167/iovs.05-0518
Tatlipinar S, Dinc UA, Yenerel NM, Gorgun E (2012) Short-term effects of a single intravitreal bevacizumab injection on retinal vessel calibre. Clin Exp Optom 95:94–98. doi:10.1111/j.1444-0938.2011.00662.x
Young TL, Anthony DC, Pierce E, Foley E, Smith LE (1997) Histopathology and vascular endothelial growth factor in untreated and diode laser-treated retinopathy of prematurity. J AAPOS 1:105–110
Penn JS, Henry MM, Tolman BL (1994) Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res 36:724–731. doi:10.1203/00006450-199412000-00007
Reynaud X, Dorey CK (1994) Extraretinal neovascularization induced by hypoxic episodes in the neonatal rat. Invest Ophthalmol Vis Sci 35:3169–3177
Zhang S, Leske DA, Holmes JM (2000) Neovascularization grading methods in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 41:887–891
Ubuka M, Sugiyama T, Onoda Y, Shiba T, Hori Y, Maeno T (2014) Changes in the blood flow of the optic nerve head induced by different concentrations of epinephrine in intravitreal infusion during vitreous surgery. Invest Ophthalmol Vis Sci 55:1625–1629. doi:10.1167/iovs.13-13801
Shiga Y, Shimura M, Asano T, Tsuda S, Yokoyama Y, Aizawa N, Omodaka K, Ryu M, Yokokura S, Takeshita T, Nakazawa T (2013) The influence of posture change on ocular blood flow in normal subjects, measured by laser speckle flowgraphy. Curr Eye Res 38:691–698. doi:10.3109/02713683.2012.758292
Sugiyama T (2014) Basic technology and clinical applications of the updated model of laser speckle flowgraphy to ocular diseases. Photo-Dermatology 1:220–234
Hida T (1982) Hyaloid vascular system of the rat: a study on its topography examined by plastic cast (author's transl). Nippon Ganka Gakkai Zasshi 86:315–327
Larrazabal LI, Penn JS (1990) Fluorescein angiography of the newborn rat. Implications in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 31:810–818
Berkowitz BA, Lukaszew RA, Mullins CM, Penn JS (1998) Impaired hyaloidal circulation function and uncoordinated ocular growth patterns in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci 39:391–396
Favazza TL, Tanimoto N, Munro RJ, Beck SC, Garcia Garrido M, Seide C, Sothilingam V, Hansen RM, Fulton AB, Seeliger MW, Akula JD (2013) Alterations of the tunica vasculosa lentis in the rat model of retinopathy of prematurity. Doc Ophthalmol 127:3–11. doi:10.1007/s10633-013-9392-z
Stahl A, Chen J, Sapieha P, Seaward MR, Krah NM, Dennison RJ, Favazza T, Bucher F, Lofqvist C, Ong H, Hellstrom A, Chemtob S, Akula JD, Smith LE (2010) Postnatal weight gain modifies severity and functional outcome of oxygen-induced proliferative retinopathy. Am J Pathol 177:2715–2723. doi:10.2353/ajpath.2010.100526
Geisen P, Peterson LJ, Martiniuk D, Uppal A, Saito Y, Hartnett ME (2008) Neutralizing antibody to VEGF reduces intravitreous neovascularization and may not interfere with ongoing intraretinal vascularization in a rat model of retinopathy of prematurity. Mol Vis 14:345–357
Rabinowitz R, Priel A, Rosner M, Pri-Chen S, Spierer A (2012) Avastin treatment reduces retinal neovascularization in a mouse model of retinopathy of prematurity. Curr Eye Res 37:624–629. doi:10.3109/02713683.2012.669003
Soetikno BT, Yi J, Shah R, Liu W, Purta P, Zhang HF, Fawzi AA (2015) Inner retinal oxygen metabolism in the 50/10 oxygen-induced retinopathy model. Sci Rep 5:16752. doi:10.1038/srep16752
Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA (2013) A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature 494:243–246. doi:10.1038/nature11823
Renz BE, Vygantas CM (1977) Hyaloid vascular remnants in human neonates. Ann Ophthalmol 9:179–184
Eller AW, Jabbour NM, Hirose T, Schepens CL (1987) Retinopathy of prematurity. The association of a persistent hyaloid artery. Ophthalmology 94:444–448
Acknowledgments
We thank Ms. E. Ozawa for her technical assistance.
This work was supported by a Toho University Tobari scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Toho University provided financial support in the form of a Tobari scholarship. The sponsor had no role in the design or conduct of this research.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Animal experiments
All procedures performed in studies involving animals were in accordance with the ethical standards of Showa University (Tokyo) (nos. #05101, #06004).
Rights and permissions
About this article
Cite this article
Matsumoto, T., Saito, Y., Itokawa, T. et al. Retinal VEGF levels correlate with ocular circulation measured by a laser speckle-micro system in an oxygen-induced retinopathy rat model. Graefes Arch Clin Exp Ophthalmol 255, 1981–1990 (2017). https://doi.org/10.1007/s00417-017-3756-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3756-0