Abstract
Background
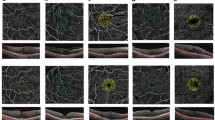
To assess changes in deep and superficial perifoveal capillary plexus after macular peeling in idiopathic and diabetic epiretinal membrane (iERM and dERM, respectively).
Methods
Cross-sectional comparative study. We included 40 eyes from 40 patients affected by iERM (20 eyes) and dERM (20 eyes), as well as 34 eyes from 17 healthy, age-matched patients. Patients received a complete ophthalmic evaluation including axial and en-face scanning spectral-domain analysis, optical coherence tomography angiography, and microperimetry. Split-spectrum amplitude-decorrelation angiography images were obtained to quantify the deep and superficial layers of perifoveal capillary-free zone (CFZ). The main outcome measures were: (i) differences at baseline between deep and superficial CFZ in iERM and dERM vs control, and (ii) changes in deep and superficial CFZ plexus after surgery in iERM vs dERM.
Results
The deep CFZ only significantly increased in dERM at the end of the follow-up period (6 months). No statistically significant differences were found between preoperative and postoperative superficial vascular plexus in iERM or dERM. At the end of the follow-up, statistically significant differences between preoperative and postoperative ganglion cell complex (GCC) average were found only in the iERM group. Best-corrected visual acuity significantly improved after surgery both in the iERM (P = 0.0053) and dERM (P < 0.0001) groups. After 6 months, macular sensitivity increased in the iERM group, but there was no statistically significant change in the dERM group.
Conclusions
In dERM, the deep CFZ significantly increases after ILM peeling, whereas postoperative angiography changes were not significant in iERM. This could be because the impaired diabetic perifoveal capillary plexus are more sensitive to the iatrogenic damage to Müller cells, induced by peeling.




Similar content being viewed by others
References
Gupta P, Yee KM, Garcia P et al (2011) Vitreoschisis in macular diseases. Br J Ophthalmol 95:376–380
Bu SC, Kuijer R, Li XR, Hooymans JM, Los LI (2014) Idiopathic epiretinal membrane. Retina 34:2317–2335
Kritzenberger M, Junglas B, Framme C et al (2011) Different collagen types define two types of idiopathic epiretinal membranes. Histopathology 58:953–965
Bringmann A, Wiedemann P (2009) Involvement of Müller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol 247:865–883
Smiddy WE, Maguire AM, Green WR et al (1989) Idiopathic epiretinal membranes. Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology 96:811–820, discussion 821
Zhao F, Gandorfer A, Haritoglou C et al (2013) Epiretinal cell proliferation in macular pucker and vitreomacular traction syndrome: analysis of flat-mounted internal limiting membrane specimens. Retina 33:77–88
Steel DH, Lotery AJ (2013) Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye 27 Suppl 1:S1–S21
Snead DR, James S, Snead MP (2008) Pathological changes in the vitreoretinal junction 1: epiretinal membrane formation. Eye 22:1310–1317
Kampik A (2012) Pathology of epiretinal membrane, idiopathic macular hole, and vitreomacular traction syndrome. Retina 32 Suppl 2:S194–S198, discussion S198–199
Junemann AG, Rejdak R, Huchzermeyer C et al (2015) Elevated vitreous body glial fibrillary acidic protein in retinal diseases. Graefes Arch Clin Exp Ophthalmol 253:2181–2186
Ripandelli G, Scarinci F, Piaggi P et al (2015) Macular pucker: to peel or not to peel the internal limiting membrane? A microperimetric response. Retina 35:498–507
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133:45–50
Di G, Weihong Y, Xiao Z et al (2015) A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol 254(5):873–879
Ishibazawa A, Nagaoka T, Takahashi A et al (2015) Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol 160:c–44.e31
Crossland MD, Dunbar HM, Rubin GS (2009) Fixation stability measurement using the MP1 microperimeter. Retina 29:651–656
Fujii GY, De Juan E Jr, Sunness J, Humayun MS, Pieramici DJ, Chang TS (2002) Patient selection for macular translocation surgery using the scanning laser ophthalmoscope. Ophthalmology 109:1737–1744
Midena E, Radin PP, Pilotto E, Ghirlando A, Convento E, Varano M (2004) Fixation pattern and macular sensitivity in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. A microperimetry study. Semin Ophthalmol 19:55–61
Scarinci F, Jampol LM, Linsenmeier RA, Fawzi AA (2015) Association of diabetic macular nonperfusion with outer retinal disruption on optical coherence tomography. JAMA Ophthalmol 133:1036–1044
Kenawy N, Wong D, Stappler T et al (2010) Does the presence of an epiretinal membrane alter the cleavage plane during internal limiting membrane peeling? Ophthalmology 117:320–323.e1
Romano MR, Vallejo-Garcia JL, Camesasca FI, Vinciguerra P, Costagliola C (2012) Vitreo-papillary adhesion as a prognostic factor in pseudo- and lamellar macular holes. Eye 26:810–815
Arend O, Wolf S, Jung F et al (1991) Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol 75:514–518
Sander B, Larsen M, Engler C, Lund-Andersen H, Parving HH (1994) Early changes in diabetic retinopathy: capillary loss and blood–retina barrier permeability in relation to metabolic control. Acta Ophthalmol 72:553–559
Pierro L, Iuliano L, Gagliardi M, Codenotti M, Ambrosi A, Bandello F (2015) Role of ganglion cell complex in visual recovery following surgical internal limiting membrane peeling. Graefes Arch Clin Exp Ophthalmol 253:37–45
Barber AJ (2003) A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 27:283–290
Lieth E, Gardner TW, Barber AJ, Antonetti DA (2000) Penn State retina research G. Retinal neurodegeneration: early pathology in diabetes. Clin Experiment Ophthalmol 28:3–8
Gella L, Raman R, Kulothungan V, Saumya Pal S, Ganesan S, Sharma T (2015) Retinal sensitivity in subjects with type 2 diabetes mellitus: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS II, Report No. 4). Br J Ophthalmol. doi:10.1136/bjophthalmol-2015-307064
Verma A, Rani PK, Raman R et al (2009) Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye 23:1824–1830
Verma A, Raman R, Vaitheeswaran K et al (2012) Does neuronal damage precede vascular damage in subjects with type 2 diabetes mellitus and having no clinical diabetic retinopathy? Ophthalmic Res 47:202–207
Lee JW, Kim IT (2010) Outcomes of idiopathic macular epiretinal membrane removal with and without internal limiting membrane peeling: a comparative study. Jpn J Ophthalmol 54:129–134
Tadayoni R, Paques M, Massin P et al (2001) Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology 108:2279–2283
Ito Y, Terasaki H, Takahashi A et al (2005) Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holes. Ophthalmology 112:1415–1420
Lazzeri S, Piaggi P, Parravano M et al (2013) Analysis of functional dissociations between best corrected visual acuity and microperimetric parameters in neovascular age-related macular degeneration patients underwent to three monthly ranibizumab injections. Clin Experiment Ophthalmol 4:4
Shahlaee A, Pefkianaki M, Hsu J, Ho AC (2016) Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol 161:50.e1–55.e1
Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L (2015) Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol 100:671–676
Acknowledgments
This paper was presented at the Vail meeting 2015 in Vail (Colorado, USA)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Competing and financial interest
The authors declare no competing and/or financial interests in the publication of this manuscript.
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Writing assistance
English language editing was provided by Malini Devadas, PhD, ELS.
Design and conduct of the study (M.R.R., G.C., GL.C.), data collection (S.S., F.S.), management (G.C., M.R.R.), analysis (G.C., C.R., GL.C.), interpretation of the data (G.C., M.R.R., GL.C.) manuscript preparation (M.R.R.), and approval of the manuscript (G.C., M.R. R., GL.C.).
Rights and permissions
About this article
Cite this article
Romano, M.R., Cennamo, G., Schiemer, S. et al. Deep and superficial OCT angiography changes after macular peeling: idiopathic vs diabetic epiretinal membranes. Graefes Arch Clin Exp Ophthalmol 255, 681–689 (2017). https://doi.org/10.1007/s00417-016-3534-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3534-4