Abstract
Purpose
To review published data pertaining to the clinical experience with a dexamethasone intravitreal implant (Ozurdex®) with a view to establishing a clinically based therapeutic regime.
Methods
A PubMed search using the MeSH terms “retinal vein occlusion” and either “pathophysiology” or “dexamethasone intravitreal implant” was undertaken for manuscripts published until August 2015. The analysis included studies involving minimally 15 patients under a prospective design or 30 under a retrospective design, a minimal follow up of 6 months, and at least 2 intravitreal Ozurdex® injections per eye.
Results
In the vast majority of eyes, satisfactory outcomes were achieved with retreatment intervals of between 3 and 5 months. Initial evidence indicates a similar efficacy compared to anti-VEGF therapies as a first-line treatment. Safety concerns associated with the long-term and repeated use of Ozurdex® are not borne out by clinical findings: its implantation is not associated with a sustained increase in intraocular pressure (IOP) over time or with the number of applications.
Conclusion
Compared with anti-VEGF therapies, the burden of retreatment is reduced. In patients with chronic macular edema not responsive to repetitive anti-VEGF therapies, the outcome after dexamethasone implant treatment is encouraging. However, these results are achieved at the expense of side effects typically associated with steroids: in up to 20 % of the Ozurdex®-treated patients, an elevation in IOP, which could be medically controlled in the majority of cases, and cataract formation or progression was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the last 10 years, intravitreal pharmacotherapy has revolutionized the therapeutic options for macular edema (ME)-associated retinal vascular diseases, and particularly for retinal vein occlusion (RVO). Substantial improvements, both visual and morphological, have been achieved under treatment with anti-VEGF agents and corticosteroids. Nevertheless, the efficacy of these intravitreal agents and their long-term outcome is still evolving. Moreover, although anti-VEGF agents can elicit a rapid regression of neovascularization, photocoagulation with a scatter laser still represents the standard of care in the prevention of extrafoveal neovascular complications whereas it remains controversial for prophylactic treatment [1]. Data that have been gleaned from randomized clinical trials regarding intravitreal therapy with ranibizumab and dexamethasone reveal significant functional improvements as compared to those that are achieved solely using laser therapy in the treatment of branch retinal vein occlusion (BRVO) and central RVO (CRVO). For the treatment of ME following RVO, intravitreal aflibercept has also been approved. However, more accurate head-to-head studies are needed to assess the relative efficacies of licensed therapies for RVO [2].
Pathophysiological aspects of the disease
In patients with RVO, ME is the most frequent cause of visual loss, irrespective of whether the macula is or is not perfused. If the visual loss that is associated with neovascularization is left untreated, vitreal haemorrhaging, tractional retinal detachment, or neovascular glaucoma can be expected to ensue in consequence of a breakdown or permanent damage to the blood–retinal barrier resulting in low-grade inflammation and relative ischemia. Therefore, an increase in the production of VEGF-A is provoked, which perpetuates vascular leakage and ME [3]. Not surprisingly, VEGF is an effective target for therapeutic intervention in vascular diseases of the retina, including CRVO [4, 5]. Anti-VEGF therapy has been demonstrated to be effective in the treatment of CRVO in several clinical trials, including CRUISE [4], HORIZON [5], GALILEO [6] and COPERNICUS [7].
The degree of ischemia is correlated with the intravitreal levels of VEGF and with the prognosis [8]. Angiographically, it can be classified according to the area of non-perfusion, nowadays, ideally using ultra-widefield technology [9, 10]. There exists no generally accepted definition for distinguishing between ischemic and non-ischemic RVO. However, from a clinical point of view, RVO may be deemed as non-ischemic (perfused) if fewer than 10 disc areas of non-perfusion are identified angiographically, which represents 75 % to 80 % of all newly diagnosed CRVO cases. The complete loss of retinal capillaries, with late venous staining in fluorescein angiography, is indicative of a significant ischemia-induced up-regulation of VEGF, and is associated with a high risk for secondary complications. If 10 or more disc areas of non-perfusion are implicated, then the risk for neovascularization of the anterior segment is heightened [9, 11]. If in this situation, the vision is good and there is no relevant ME, then anti-VEGF therapy would not be recommended, but the ischemic areas should be subjected to careful and, in the early phase, timely observation and eventually peripheral panretinal laser photocoagulation to avoid the risk of neovascular complications. It is worthy of note that treatment with an anti-VEGF agent can mask the degree of underlying ischemia [1, 2].
Although the mean vitreal levels of VEGF are elevated in both disease states (CRVO and BRVO), in one-third of the eyes, these may fall within the normal range despite the presence of ME [8, 12, 13]. This finding points to the existence of VEGF-independent pathways that can drive ME and could be the reason that a fraction of patients is less responsive to anti-VEGF therapy alone.
RVO is not an isolated retinal vascular disease; choroidal involvement is part of the pathology [14] and pigment epithelial detachment is present in up to one third of the patients.
The dexamethasone-based intravitreal implant (Ozurdex®)
Ozurdex® is a dexamethasone-bearing vehicle, which has been developed in the form of a biodegradable intravitreal implant and which delivers a 700-μg dose of the drug to the retina and the vitreous. It has been approved for use in the treatment of RVO-associated ME [24] and non-infectious posterior uveitis [16] by the Federal Office of Food and Drug Administration in the USA (FDA), by the Commission for European Medicines Administration (EMA), and by Swissmedic. Ozurdex® has also been used efficaciously in the treatment of other clinical conditions including the postoperative ME that is associated with cataract surgery (Irvine-Gass syndrome) and vitrectomy, diabetic ME, persistent ME, intra-ocular inflammation and adjunctively in cases of age-related macular degeneration [15]. However, randomized clinical trials relating to the use of Ozurdex® in the treatment of these conditions have as yet not all been completed. Safety concerns associated to the use of Ozurdex® include the formation or progression of cataracts and a transient increase in intraocular pressure that peaks after 1 to 2 months, yet is usually amenable to medical management [15, 17, 18].
Recently, it was shown by aqueous laser flare measurements that the impact of Ozurdex® on recovery of the uveovascular barrier in eyes with RVO correlates with its effect on visual acuity and central retinal thickness [19].
Published data appertaining to the effects of steroids on RVO
In the SCORE study — a multicenter clinical trial — the efficacy and safety of 1-mg and 4-mg doses of preservative-free intravitreal triamcinolone were assessed in patients with ME that had developed secondary to perfused CRVO. This study marked a turning point in the management of RVO, since it was the first of its kind to report on an effective treatment strategy for CRVO-associated ME. For BRVO-associated ME, no differences in visual acuity were observed between the standard care (laser treatment) and the triamcinolone-treated groups. However, the adverse event rates — particularly for rises in intraocular pressure and the formation or progression of cataracts — were higher in the group of BRVO patients that had been treated with 4-mg doses of triamcinolone than in either the BRVO-patient group that had received 1-mg doses of the drug or the standard care one [20, 21].
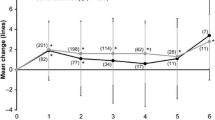
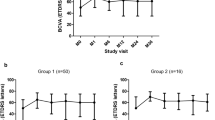
In the Geneva study — a randomized, controlled, clinical trial — 1267 patients with ME, which had developed secondary to either CRVO (35 %) or BRVO (65 %), were monitored for minimally 12 months after treatment with 0.35- and 0.7-mg doses of dexamethasone [22, 23]. The dexamethasone-treated eyes manifested significant improvements in visual function in contrast to the untreated controls, and they sustained a supportable profile of side effects. A sub-group of 17 patients were monitored for a minimal follow-up period of 50 months. The data that were gleaned from these individuals revealed the visual prognosis to be better in BRVO- than in CRVO-afflicted eyes. In more than 50 % of these patients, a cataract progression was observed. However, a persistent rise in intraocular pressure was reported in only one case; and, likewise, in one case alone, neovascularization and vitreal haemorrhaging were apparent. Hence, treatment with dexamethasone may be deemed safe in the long run [22, 23]. A limitation of the Geneva study was that only two injections of dexamethasone at fixed 6-monthly intervals were administered. During the intervening period, the visual acuity deteriorated and the retinal thickness increased substantially, owing to the subsidence of drug activity. This problem was avoided in eyes that underwent anti-VEGF therapy, since the drug was delivered on a monthly basis in this study [23]. From the body of data that has been collected to date, it is now evident that the effects of intravitreally-administered dexamethasone can be sustained for 4 months (range: 3 to 7 months) irrespective of the patient’s clinical background. A retreatment initiation on an as needed basis (PRN regime) would necessitate reinjection intervals of substantially less than 6 months for the vast majority of eyes [24, 25–27]. Since the recurrence of retinal edema precedes the functional impairment by 2 weeks, the decision for reinjection would ideally be based on OCT criteria [28].
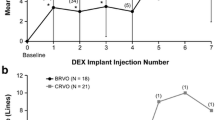
In the Shasta study — a multicenter retrospective clinical trial — 289 patients with CRVO- or BRVO-associated ME were evaluated after treatment with dexamethasone. Two to 9 dexamethasone implants (mean: 3.2) were injected either as the sole therapy (29.1 %) or in conjunction with other treatment strategies. The mean duration of the ME prior to the injection of the first dexamethasone implant was 18.4 months, and the mean re-injection interval was 5 to 6 months. A best-corrected visual acuity increase of +1.0 line was attained 4 weeks after the onset of treatment, and this level was subsequently sustained for 20 weeks after the injection of the final dexamethasone implant. The central retinal thickness decreased significantly compared to the baseline level (P ≤ 0.037) and a best-corrected visual acuity increase of ≥ 2 lines was achieved in 66.7 % of the patients with CRVO and in 59.7 % of those with BRVO. In 32.6 % of the CRVO- and BRVO-afflicted eyes, an intraocular pressure increase of ≥ 10 mm Hg was reported. In 29.1 % of the patients, intraocular pressure-lowering medication was administered, and in 1.7 %, incisional glaucoma surgery was called for. In this study, a larger cohort of RVO patients receiving more than two dexamethasone-bearing implants was assessed than heretofore, and no new safety concerns were identified as a consequence of the multiple injections [29, 30]. In an earlier published retrospective assessment of 33 RVO-afflicted eyes, re-treatment with dexamethasone was necessary 4.7 ± 1.1 months after the first injection and 5.1 ± 1.5 months after the second in order to sustain a significant improvement in the best-corrected visual acuity and in the central retinal thickness. No side effects other than those to be expected after the intraocular administration of corticosteroids were revealed [31]. Functional improvements were evident already after the 1st month of treatment with dexamethasone, and were thereafter sustained until the end of the 3rd month; but from the 4th month onward, they were lost. The repeated injection of dexamethasone over a time course exceeding 12 months is deemed to be a safe procedure, even though it is associated with accelerated cataract progression and rises in intraocular pressure which, nevertheless, can usually be brought under control by medical management [26, 32]. In a retrospective study involving a consecutive series of 51 eyes of 49 patients with RVO-associated ME, 70 % of the individuals responded to injections of dexamethasone implants with an improvement in visual acuity and a recession in ME within 3 months of treatment, and in 30 % of the eyes, the gain in visual acuity was ≥15 letters. In 56 % of the patients, a relapse was observed after a median follow-up time of 17 to 18 weeks, and re-injections of dexamethasone were required to restore the gains in visual acuity. However, in some of the eyes, the duration of the positive response to treatment was curtailed to 10 weeks. In 27 % of the eyes, the rises in intraocular pressure needed to be medically controlled, and in 5 %, neovascular complications developed [24]. The findings appertaining to rises in intraocular pressure accord well with the data that are presented in another study, which involved the retrospective analysis of 342 RVO-afflicted eyes, that had been monitored for 8 months after treatment with intravitreal injections of dexamethasone [33]. In 20 % of the eyes, the intraocular pressure increased; in 9 %, it rose by more than 10 mm Hg above the baseline level, but in only 2 % did the rise exceed 35 mm Hg. Eyes in which a pre-existing glaucomatous condition had been identified were not more prone to increases in intraocular pressure than were those in which no such state had been registered. During the 8-month follow-up period, 1.5 % of the eyes required cataract surgery. No correlation has been observed between the number of dexamethasone injections, neither the time interval to re-injection, nor rises in intraocular pressure. Hence, it is unclear why the increases in intraocular pressure were less pronounced in the aforementioned German study [33] than in the North American Shasta trial [29, 30]. So, the findings of the latter study are more akin to those that have been reported for similarly afflicted eyes in Switzerland.
Two case studies in which anti-VEGF- and dexamethasone-based therapies were combined have been reported [34, 35]. In one of these — a retrospective interventional study — 33 RVO-afflicted eyes were intravitreally injected either with ranibizumab and then with dexamethasone, or with dexamethasone alone. The visual gains were more marked and were achieved more rapidly in the former than in the latter group [34]. In another prospective study, 34 RVO-afflicted eyes were intravitreally injected either with a combination of bevacizumab and dexamethasone, or with dexamethasone alone. The combined therapeutic regime proved to be more efficacious than the mono-therapeutic one with increase in visual acuity and reduction of central retinal thickness. The visual gains that were achieved persisted for longer periods of time [35]. This confirms the results of the Shasta trial in which combination therapy showed an extended duration of effect under Ozurdex if combined with anti-VEGF drugs. The risk of an increased IOP was higher in the combination therapy group, which may at least partially be explained by a selection bias [30].
Though the possibility of tachyphylaxis after repeated injections of intraocular corticosteroids has, meanwhile, been discussed for a decade [24, 36, 37], there is still no evidence for its existence after repeated use of intravitreal corticosteroids [26, 38, 39]. A rebound phenomenon, in contrast to tachyphylaxis, representing a more pronounced macular edema after loss of therapeutic effect has been reported [32, 40] and seems to be similar to that after repeated anti-VEGF injections in RVO [41, 42].
The efficacy of dexamethasone implants in cases with refractory macular edema under anti-VEGF therapy for retinal vascular disorders has been demonstrated [43]. Nevertheless, eyes pre-treated with anti-VEGF drugs, i.e. bevacizumab, seem to respond anatomically equally well to an additional therapy with triamcinolone or dexamethasone, although this does not obviously go along with an additional gain in visual function compared to the one in treatment-naïve eyes. The former, however, is prone to a more pronounced effect on the IOP of affected eyes, whereas the latter showed a moderately longer duration of effect [44].
The dexamethasone implant may be as effective as ranibizumab in treatment-naïve eyes with vision loss due to RVO [45, 46], requiring less injections for the well-known price of a medically well-controlled rise in IOP of more than 5 mmHg and cataract progression in nearly half of the eyes. On the other hand, change of treatment is less likely required after intravitreal dexamethasone than after anti-VEGF therapy [30, 47–50]. The dexamethasone implant may be used in cases in which the role of anti-VEGF agents has not been equally established, namely in ischemic retinopathies: its positive — though due to the ischaemic state, functionally limited — effect over 12 months in ischaemic RVO has prospectively been demonstrated [51, 52]. In individuals younger than 50 years with 50 % experiencing a three-line improvement in visual acuity with a mean of 1.8 implantations over 12 months was achieved. This is worth mentioning because these patients comprise the working-age group suffering mostly from the economic burden of frequent controls and reinjections [53].
Finally, the risk of complications, namely of an increase of IOP, does peak at 1 to 2 months after first treatment. Thereafter, no rise over time or with the number of injections was reported, although patients with pre-existing ocular hypertension and glaucoma may be exposed to a higher risk. An initial control of IOP will be needed within 4 weeks after implantation. Phakic patients have to expect cataract progression with the need of cataract surgery within roughly 1 year [54, 55].
As a therapeutic weapon against ME of various aetiologies, dexamethasone is assuming an increasingly elevated rank in the clinical arsenal, owing to its potency, the dose-consistency, the prolonged duration of action, and, compared to other corticosteroids, its favourable safety profile. However, few available prospective head-to-head comparisons and combinations with other treatment modalities allow as of yet one to precisely define the role of dexamethasone in clinical practice [15] (Table 1).
Conclusion
To achieve satisfactory visual and anatomic outcomes after the intravitreal administration of dexamethasone implants, the re-injection interval needs to be considerably less than 6 months in many instances. To date, there are no indications that the long-term use of dexamethasone poses a safety hazard. Further evidence appertaining thereto has been recently furnished by a phase III trial (MEAD) in which diabetic ME was treated with intravitreal injections of dexamethasone implants. The findings revealed the lack of correlation between dexamethasone treatments per se and intermittent increases in intraocular pressure, or between the number of dexamethasone injections and rises in intraocular pressure per se [56]. In existing head-to-head trials in which the effects of anti-VEGF agents and dexamethasone have been directly compared, the interval of 6 months was, according to more recent experience, too protracted for the sustenance of the therapeutic effect, which can be maintained for approximately 4 months.
Published reports in which re-injections have been made after shorter intervals on an “as needed” basis are now available [26, 32]. Compared to anti-VEGF therapies, the burden of re-treatment is reduced. And in cases of chronic ME that are refractive to repeated anti-VEGF therapy, the response to dexamethasone is good. The future will reveal whether an early combined treatment strategy involving an anti-VEGF agent and dexamethasone elicits superior results to those that can be achieved using either of these drugs alone in terms of a long-term compensation of the underlying impaired vascular situation [34, 35].
References
Hahn P, Fekrat S (2012) Best practices for treatment of retinal vein occlusion. Curr Opin Ophthalmol 23(3):175–81
Glanville J, Patterson J, McCool R et al (2014) Efficacy and safety of widely used treatments for macular oedema secondary to retinal vein occlusion: a systematic review. BMC Ophthalmol 14:7
Wong TY, Scott IU (2010) Clinical practice, retinal-vein occlusion. N Engl J Med 363(22):2135–2144
Campochiaro PA, Brown DM, Awh CC et al (2011) Sustained benefits from Ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 118(10):2041–2049
Heier JS, Campochiaro PA, Yau L et al (2012) Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology 119(4):802–809
Holz FG, Roider J, Ogura Y et al (2013) VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol 97(3):278–284
Brown DM, Heier JS, Clark WL et al (2013) Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 155(3):429–437
Noma H, Funatsu H, Mimura T et al (2009) Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology 116(1):87–93
Nguyen NX, Küchle M (1993) Aqueous flare and cells in eyes with retinal vein occlusion--correlation with retinal fluorescein angiographic findings. Br J Ophthalmol 77:280–3
Tan CS, Chew MC, van Hemert J, Singer MA, Bell D, Sadda SR (2015) Measuring the precise area of peripheral retinal non-perfusion using ultra-widefield imaging and its correlation with the ischaemic index. Br J Ophthalmol.; pii:bjophthalmol-2015-306652
The Central Vein Occlusion Study Group (1993) Baseline and early natural history report. Arch Ophthalmol 111(8):1087–1095
Noma H, Minamoto A, Funatsu H, Tsukamoto H, Nakano K, Yamashita H, Mishima HK (2006) Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 244(3):309–15
Koss MJ, Pfister M, Rothweiler F, Michaelis M, Cinatl J, Schubert R, Koch FH (2012) Comparison of cytokine levels from undiluted vitreous of untreated patients with retinal vein occlusion. Acta Ophthalmol 90(2):e98–e103
Lee EK, Han JM, Hyon JY, Yu HG (2015) Changes in choroidal thickness after intravitreal dexamethasone implant injection in retinal vein occlusion. Br J Ophthalmol.; pii: bjophthalmol-2014-306417
Glacet-Bernard A, Coscas G, Zourdani A et al. (2011) Steroids and macular edema from retinal vein occlusion. Eur J Ophthalmol.;21 Suppl 6:S37-44
Whitcup SM, Robinson MR (2015) Development of a dexamethasone intravitreal implant for the treatment of noninfectious posterior segment uveitis. Ann N Y Acad Sci. doi:10.1111/nyas.12824
Coscas G, Augustin A, Bandello F et al (2014) Retreatment with Ozurdex for macular edema secondary to retinal vein occlusion. Eur J Ophthalmol 24(1):1–9
Mayer WJ, Wolf A, Kernt M et al (2013) Twelve-month experience with Ozurdex for the treatment of macular edema associated with retinal vein occlusion. Eye (Lond) 27(7):816–22
Demirel S, Yanık Ö, Batıoglu F, Özmert E, Bas Z (2015) Aqueous flare as an indicator of response to Dexamethasone treatment in retinal vein occlusions: a pilot study. Curr Eye Res 3:1–8
Ip MS, Scott IU, VanVeldhuisen PC, Study Research Group SCORE et al (2009) A randomized trial comparing the efficacy and safety of Intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 5. Arch Ophthalmol 127(9):1101–14
Scott IU, Ip MS, Van Veldhuisen PC, Study Research Group SCORE et al (2009) A randomized trial comparing the efficacy and safety of Intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the standard care vs corticosteroid for retinal vein occlusion (SCORE) study report 6. Arch Ophthalmol 127(9):1115–28
Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J, Li XY, Whitcup SM, Li J, Ozuredx GENEVA Study Group (2011) Dexamethasone Intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 118(12):2453–60
London NJ, Chiang A, Haller JA (2011) The dexamethasone drug delivery system: indications and evidence. Adv Ther 28(5):351–66
Joshi L, Yaganti S, Gemenetzi M, Lightman S, Lindfield D, Liolios V, Menezo V, Shao E, Taylor SR (2013) Dexamethasone implants in retinal vein occlusion: 12-month clinical effectiveness using repeat injections as-needed. Br J Ophthalmol 97(8):1040–4
Augustin AJ, Holz FG, Haritoglou C, Mayer WJ, Bopp S, Scheuerle AF, Maier M, Sekundo W, Sandner D, Shirlaw A, Hattenbach LO (2015) Retrospective, observational study in patients receiving a dexamethasone intravitreal implant 0.7 mg for macular oedema secondary to retinal vein occlusion. Ophthalmologica 233:18–26
Bandello F, Parravano M, Cavallero E, Cascavilla ML, Triolo G, Querques L, Borrelli E, Giorno P, Varano M, Lattanzio R, Querques G (2015) Prospective evaluation of morphological and functional changes after repeated intravitreal dexamethasone implant (Ozurdex®) for retinal vein occlusion. Ophthalmic Res 53:207–16
Bezatis A, Spital G, Höhn F, Maier M, Clemens CR, Wachtlin J, Lehmann F, Hattenbach LO, Feltgen N, Meyer CH (2013) Functional and anatomical results after a single Intravitreal ozurdex injection in retinale vein occlusion: a 6-month follow-up- the SOLO study. Acta Opthalmol 91(5):e340–7
Fortoul V, Denis P, Kodjikian L (2015) Anatomical and functional recurrence after Dexamethasone Intravitreal implants: a 6-month prospective study. Eye (Lond) 29:769–75
Capone A Jr, Singer MA, Dodwell DG et al (2014) Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina 34(2):342–51
Singer MA, Capone A Jr, Dugel PU, Dreyer RF, Dodwell DG, Roth DB, Shi R, Walt JG, Scott LC, Hollander DA, SHASTA Study Group (2015) Two or more dexamethasone intravitreal implants as monotherapy or in combination therapy for macular edema in retinal vein occlusion: subgroup analysis of a retrospective chart review study. BMC Ophthalmol 15:33
Querques L, Querques G, Lattanzio R et al (2013) Repeated intravitreal dexamethasone implant (Ozurdex®) for retinal vein occlusion. Ophthalmologica 229(1):21–5
Querques G, Cascavilla ML, Cavallero E et al (2014) Changes in macular function after ozurdex for retinal vein occlusion. Optom Vis Sci 91(7):760–8
Schmitz K, Maier M, Clemens CR, Group GRVO et al (2014) Reliability and safety of Intravitreal ozurdex injections. The ZERO study. Ophthalmologe 111(1):44–52
Iu LP, Zhao P, Yeung IY et al. (2014) Sequential therapy with ranibizumab and dexamethasone intravitreal implant is better than dexamethasone monotherapy for macular oedema due to retinal vein occlusion. Br J Ophthalmol.;pii: bjophthalmol-2014-305661
Singer MA, Bell DJ, Woods P et al (2012) Effect of combination therapy with Bevacizumab and Dexamethasone Intravitreal implant in patients with retinal vein occlusion. Retina 32(7):1289–94
Jonas JB, Spandau UH, Kamppeter BA, Vossmerbaeumer U, Harder B, Sauder G (2006) Repeated Intravitreal high-dosage injections of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology 113(5):800–4
Parodi MB, Iacono P, Campa C, La Spina C, Triolo G, Lattanzio R, Bandello F (2014) Dexamethasone tachyphylaxis in the treatment of macular oedema. Acta Ophthalmol 92(3):e243–4
Matonti F, Hoffart L, Baeteman C, Denis D (2012) Repeated treatment for macular edema in vein occlusion by intravitreal implant of dexamethasone. Case Rep Ophthalmol 3(3):339–42
Unsal E, Eltutar K, Sultan P, Gungel H (2015) The efficiency of Intravitreal Dexamethasone implants in the treatment of macular edema secondary to retinal vein occlusion. J Ocul Pharmacol Ther 31(6):350–6
Parodi MB, Iacono P, De Benedetto U, Cascavilla M, Bandello F (2012) Rebound effect after intravitreal dexamethasone implant for the treatment of macular edema secondary to central retinal vein occlusion. J Ocul Pharmacol Ther 28(6):566–8
Marticorena J, Romano MR, Heimann H, Stappler T, Gibran K, Groenewald C, Pearce I, Wong D (2011) Intravitreal bevacizumab for retinal vein occlusion and early growth of epiretinal membrane: a possible secondary effect? Br J Ophthalmol 95(3):391–5
Hung KH, Lee SM, Lee SY, Lee FL, Yang CS (2010) Intravitreal bevacizumab (avastin) in the treatment of macular edema associated with perfused retinal vein occlusion. J Ocul Pharmacol Ther 26(1):85–90
Alshahrani ST, Dolz-Marco R, Gallego-Pinazo R, Diaz-Llopis M, Arevalo JF; KKESH International Collaborative Retina Study Group (2015) intravitreal dexamethasone implant for the treatment of refractory macular edema in retinal vascular diseases: Results of the KKESH International Collaborative Retina Study Group. Retina
Ozkok A, Saleh OA, Sigford DK, Heroman JW, Schaal S (2015) The Omar study: comparison of ozurdex and triamcinolone acetonide for refractory cystoid macular edema in retinal vein occlusion. Retina 35(7):1393–400
Gado AS, Macky TA (2014) Dexamethasone intravitreous implant versus bevacizumab for central retinal vein occlusion-related macular oedema: a prospective randomized comparison. Clin Experiment Ophthalmol 42(7):650–5
Matonti F, Meyer F, Guigou S et al (2013) Ozurdex in the management of the macular edema following retinal vein occlusion in clinical practice. Acta Ophthalmol 91(7):e584–6
Mayer WJ, Hadjigoli A, Wolf A, Herold T, Haritoglou C (2015) Comparison of Intravitreal Dexamethasone Implant versus Intravitreal Ranibizumab as a First-Line Treatment of Macular Oedema due to Retinal Vein Occlusion. Klin Monatsbl Augenheilkd
Yumusak E, Buyuktortop N, Ornek K (2015) Early results of dexamethasone implant, ranibizumab, and triamcinolone in macular edema due to branch retinal vein occlusion. Eur J Ophthalmol.;11:0
Chiquet C, Dupuy C, Bron AM, Aptel F, Straub M, Isaico R, Romanet JP, Creuzot-Garcher C (2015) Intravitreal dexamethasone implant versus anti-VEGF injection for treatment-naïve patients with retinal vein occlusion and macular edema: a 12-month follow-up study. Graefes Arch Clin Exp Ophthalmol
Nghiem-Buffet S, Fajnkuchen F, Buffet M, Ayrault S, Le Gloahec-Lorcy A, Grenet T, Delahaye-Mazza C, Quentel G, Cohen SY (2014) Intravitreal ranibizumab and/or dexamethasone implant for macular edema secondary to retinal vein occlusion. Ophthalmologica 232(4):216–22
Parodi MB, Iacono P, Petruzzi G, Parravano M, Varano M, Bandello F (2015) Dexamethasone implant for macular edema secondary to ischemic retinal vein occlusions. Retina 35(7):1387–92
Maggio E, Polito A, Guerriero M, Pertile G (2014) Intravitreal dexamethasone implant for macular edema secondary to retinal vein occlusion: 12-month follow-up and prognostic factors. Ophthalmologica 232(4):207–15
Battaglia Parodi M, Iacono P, Sacconi R, Parravano M, Varano M, Bandello F (2015) Dexamethasone implant for macular edema secondary to central retinal vein occlusion in patients younger than 50 years. Retina 35(7):1381–6
Reid GA, Sahota DS, Sarhan M (2015) Observed complications from dexamethasone intravitreal implant for the treatment of macular edema in retinal vein occlusion over 3 treatment rounds. Retina 35(8):1647–55
Caillaux V, Valtot F, Souied EH, Mimoun G (2015) Intraocular pressure after intravitreal injection of dexamethasone implant for macular edema resulting from retinal vein occlusion. Eur J Ophthalmol.;30;25(5):454–8
Boyer DS, Yoon YH, Belfort R Jr, Ozurdex MEAD Study Group et al (2014) Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121(10):1904–14
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Human and animal rights and informed consent
For this type of study, formal consent is not required.
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Garweg, J.G., Zandi, S. Retinal vein occlusion and the use of a dexamethasone intravitreal implant (Ozurdex®) in its treatment. Graefes Arch Clin Exp Ophthalmol 254, 1257–1265 (2016). https://doi.org/10.1007/s00417-016-3350-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3350-x