Abstract
Purpose
The purpose of this study was to evaluate the ocular distribution of intravenously administered tigecycline in a rabbit uveitis model.
Methods
Tigecycline, which has a broad spectrum of activity against many gram-positive, gram-negative, and anaerobic organisms, was given intravenously to rabbits at 7 mg/kg of body weight starting 24 h after induction of uveitis by intravitreal endotoxin injection. Tigecycline concentrations were determined by high performance liquid chromatography-mass spectrometry (LC-MS/MS) assay in the aqueous humor, vitreous humor, and plasma 1, 3, 6, and 24 h after administration of a single dose.
Results
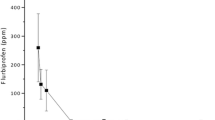
The maximum concentrations were found within 1 h after the end of the intravenously given tigecycline, and were 1,308.60 ± 301.76 ng/mL in plasma, 181.40 ± 51.32 ng/mL in vitreous humor and 145.00 ± 55.29 ng/mL in aqueous humor of the inflamed eye. After 24 h, no drug was detectable in the aqueous and vitreous of the normal eyes, whereas small amounts of drug were detectable in inflamed eyes and in plasma.
Conclusions
Tigecycline did not reach therapeutically significant levels in the aqueous and the vitreous humor of rabbit eyes. The findings suggest a limited role for intravenously administered tigecycline in the treatment of bacterial endophthalmitis.




Similar content being viewed by others
References
Callegan MC, Engelbert M, Parke DW 2nd, Jett BD, Gilmore MS (2002) Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin Microbiol Rev 15:111–124
Scott IU, Flynn HW Jr, Feuer W, Pflugfelder SC, Alfonso EC, Forster RK, Miller D (1996) Endophthalmitis associated with microbial keratitis. Ophthalmology 103:1864–1870
Greer ND (2006) Tigecycline (tygacil): the first in the glycylcycline class of antibiotics. Proc (Baylor Univ Med Cent) 19:155–161
Zhanel GG, Homenuik K, Nichol K, Noreddin A, Vercaigne L, Embil J, Gin A, Karlowsky JA, Hoban DJ (2004) The glycylcyclines: a comparative review with the tetracyclines. Drugs 64:63–88
Gary ES, William AC (2006) Tigecycline: a critical analysis. CID 43:518–524
Daily MJ, Peyman GA, Fishman G (1973) Intravitreal injection of methicillin for treatment of endophthalmitis. Am J Ophthalmol 76:343–350
Jay WM, Shockley RK (1988) Toxicity and pharmacokinetics of cefepime (BMY-28142) following intravitreal injection in pigmented rabbit eyes. J Ocul Pharmacol 4:345–349
Wiechens B, Neumann D, Grammer JB, Pleyer U, Hedderich J, Duncker GI (1998) Retinal toxicity of liposome-incorporated and free ofloxacin after intravitreal injection in rabbit eyes. Int Ophthalmol 22:133–143
Endophthalmitis Vitrectomy Study Group (1995) Results of the endophthalmitis vitrectomy study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 113:1479–1496
Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG (2005) Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev 57:2010–2032
Muralidharan G, Micalizzi M, Speth J, Raible D, Troy S (2005) Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob Agents Chemother 49:220–229
Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ (2006) Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother 58:1221–1229
Lefort A, Lafaurie M, Massias L, Petegnief Y, Saleh-Mghir A, Muller-Serieys C, Le Guludec D, Fantin B (2003) Activity and diffusion of tigecycline (GAR-936) in experimental enterococcal endocarditis. Antimicrob Agents Chemother 47:216–222
Abel R Jr, Boyle GL (1976) Dissecting ocular tissue for intraocular drug studies. Invest Ophthalmol 15:216–219
Pai MP (2014) Serum and urine pharmacokinetics of tigecycline in obese class III and normal weight adults. J Antimicrob Chemother 69:190–199
Crandon JL, Kim A, Nicolau DP (2009) Comparison of tigecycline penetration into the epithelial lining fluid of infected and uninfected murine lungs. J Antimicrob Chemother 64:837–839
Dandache P, Nicolau DP, Sakoulas G (2009) Tigecyline for the treatment of multidrug-resistant Klebsiella pneumoniae meningitis. Infect Dis Clin Pract 17:66–68
Wadi JA, Al Rub MA (2007) Multidrug resistant acinetobacter nosocomial meningitis treated successfully with parenteral tigecycline. Ann Saudi Med 27:456–458
Mannermaa E, Vellonen KS, Urtti A (2006) Drug transport in corneal epithelium and blood-retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev 58:1136–1163
Ferencz JR, Assia EI, Diamantstein L, Rubinstein E (1999) Vancomycin concentration in the vitreous after intravenous and intravitreal administration for postoperative endophthalmitis. Arch Ophthalmol 117:1023–1027
Nau R, Sörgel F, Eiffert H (2010) Penetration of drugs through the blood-cerebrospinal fluid/blood barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883
Romero CF, Rai MK, Lowder CY, Adal KA (1999) Endogenous endophthalmitis: case report and brief review. Am Fam Physician 60:510–514
Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG (2005) Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol 139:135–140
Acknowledgments
This work was supported by grants from Konya Training and Research Hospital (KTRH-28), Konya, Turkey.
Author disclosure statement
The authors have no financial or proprietary interest in any material or method mentioned. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozcimen, M., Sakarya, Y., Ozcimen, S. et al. Pharmacokinetics of intravenously administered tigecycline in eye compartments: an experimental study. Graefes Arch Clin Exp Ophthalmol 252, 1993–1997 (2014). https://doi.org/10.1007/s00417-014-2784-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2784-2