Abstract
Introduction
Azithromycin demonstrates high tissue distribution and prolonged elimination half-life. In this study, we monitored the pharmacokinetics of a single ophthalmic administration of 1% azithromycin ophthalmic solution containing polycarbophil in the extraocular tissues, including the eyelid, and compared it with that of two commercial ophthalmic products, 1.5% levofloxacin ophthalmic solution and 0.3% ofloxacin ophthalmic ointment.
Methods
Rabbits were treated with either a single topical administration of 1% azithromycin ophthalmic solution, 1.5% levofloxacin ophthalmic solution, or 0.3% ofloxacin ophthalmic ointment. The eyelid, conjunctiva, and cornea were collected at 0.25, 0.5, 1, 2, 4, 8, and 24 h post-administration. Tissue samples were pretreated for drug concentration measurements by ultra-performance liquid chromatography-tandem mass spectrometry. Pharmacokinetic parameters were determined by non-compartmental analysis.
Results
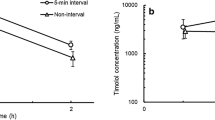
Azithromycin was rapidly absorbed, and its levels remained near the observed maximum concentrations for up to 24 h post-administration in all tissue. In contrast, extraocular tissue concentrations of levofloxacin and ofloxacin decreased with time. The maximum concentrations of azithromycin, levofloxacin, and ofloxacin were 35.6, 34.1, and 55.1 µg/g in the eyelid, 44.2, 46.8, and 20.4 µg/g in the conjunctiva, and 79.9, 18.0, and 2.21 µg/g in the cornea, respectively. The values of the area under the curve from 0 to 24 h after administration of azithromycin, levofloxacin, and ofloxacin were 602, 58.5, and 267 µg·h/g in the eyelid, 837, 43.2, and 51.9 µg·h/g in the conjunctiva, and 1250, 26.4, and 5.46 µg h/g in the cornea, respectively.
Conclusion
The drug concentrations of azithromycin and levofloxacin were relatively comparable among the extraocular tissues following topical administration of the respective ophthalmic solutions, whereas the concentrations of ofloxacin varied following dosing of its ophthalmic ointment. The slow elimination profile in any extraocular tissue of rabbits was unique to azithromycin, and led to the demonstration of high exposures of azithromycin in all extraocular tissues after ophthalmic administration.
Funding
This research and Rapid Service Fees were supported by Senju Pharmaceutical Co., Ltd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extraocular tissues such as the eyelid, conjunctiva, and cornea are exposed to a variety of exogenous materials and microorganisms, including pathogenic bacteria and viruses. Infectious diseases including blepharitis, conjunctivitis, and keratitis are caused by pathogenic bacteria [1,2,3,4,5], which are typically treated with topical formulations of antimicrobial agents [6, 7].
Azithromycin is a broad-spectrum macrolide antibiotic with anti-inflammatory properties. A formulation of 1% azithromycin ophthalmic solution (AzaSite® 1% sterile topical ophthalmic drops) approved by the Food and Drug Administration is commercially available in the United States for the treatment of conjunctivitis. In addition, a 2009 US survey on blepharitis found that azithromycin was most frequently prescribed as treatment for this ophthalmologic condition by both ophthalmologists and optometrists [8]. In Japan, a formulation of 1% azithromycin ophthalmic solution (Azimycin® ophthalmic solution 1%) containing the same ingredients as AzaSite® was recently approved for treatment of extraocular infectious diseases including conjunctivitis and blepharitis.
The 1% azithromycin ophthalmic solution includes a polymeric ocular drug delivery system based on polycarbophil (DuraSite®). Topical administration of 1% azithromycin ophthalmic solution with polycarbophil led to a higher azithromycin concentration in the ocular tissues, including the eyelid, compared with that observed after administering a formulation without polycarbophil [9]. Moreover, azithromycin has shown prolonged elimination half-life in ocular tissues [9, 10].
In Japan, 1.5% levofloxacin ophthalmic solution (Cravit® ophthalmic solution 1.5%) and 0.3% ofloxacin ophthalmic ointment (Tarivid® ophthalmic ointment 0.3%) are commercially available. Although these formulations are indicated for treatment of blepharitis, their concentration profiles in the eyelid after topical administration have not been reported.
In this report, we assess the pharmacokinetics of azithromycin in the extraocular tissues, including the eyelid, after a single topical dose of 1% azithromycin ophthalmic solution containing polycarbophil to rabbits, which we compare with that of levofloxacin and ofloxacin in these tissues after a single topical administration of the respective ophthalmic formulations.
Methods
Animals
A total of 42 male Japanese White Rabbits (2.0–2.5 kg) were obtained from Kitayama Labes (Ina, Japan) and housed individually in cages under controlled environmental conditions with ad libitum access to water and fed 100 g dry pellet once daily. All animals were managed in accordance with the local standard operating procedures in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All institutional and national guidelines for the care and use of laboratory animals were followed. The study protocol, including all experimental procedures, was approved by the Institutional Animal Care and Use Committee of Senju Pharmaceutical Co.
Drugs
The 1% azithromycin ophthalmic solution containing polycarbophil and other ingredients was prepared according to the formulation approved in Japan. The 1.5% levofloxacin ophthalmic solution (Cravit® ophthalmic solution 1.5%; Santen Pharmaceutical Co., Osaka, Japan) and the 0.3% ofloxacin ophthalmic ointment (Tarivid® ophthalmic ointment 0.3%; Santen Pharmaceutical Co., Osaka, Japan) were commercially available.
Drug Administration and Ocular Sample Collection
Ophthalmic dosing of any two of the following were applied to the eye of each animal (n = 4 eyes per sampling time point): a single dose of 30 µL of 1% azithromycin ophthalmic solution, 30 µL of 1.5% levofloxacin ophthalmic solution, or 46 µL (approximately 40 mg) of 0.3% ofloxacin ophthalmic ointment. At predetermined time points of 0.25, 0.5, 1, 2, 4, 8, and 24 h after dosing, animals were euthanized with more than 100 mg/kg of pentobarbital (intravenous administration), and ocular samples were surgically collected and separated into the eyelid, conjunctiva, and cornea.
Analysis of Drug Concentration
Ocular tissues were pretreated for quantification of each drug using an ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) system. Each tissue was homogenized with zirconia balls in water using a beads homogenizer (Precellys® Evolution; Bertin Technologies, France). The homogenates were recovered and further pretreated by solid-phase extraction using an Oasis® HLB µElution plate (Waters Corporation, Milford, MA, USA). The UPLC-MS/MS system consisted of a UPLC instrument (Waters Corporation, Milford, MA, USA) and an API 4000 triple quadrupole mass spectrometer (AB SCIEX, Framingham, MA, USA). Phenytoin (FUJIFILM, Wako Pure Chemical Corporation, Osaka, Japan) was used as an internal standard for all analytes, and the analytes and the internal standard were detected in positive ion mode. Lower limits of quantification for azithromycin were 0.0220, 0.0825, and 0.0275 μg/g for the eyelid, conjunctiva, and cornea, respectively, and those for levofloxacin and ofloxacin were 0.0132, 0.0165, and 0.0132 μg/g for the eyelid, conjunctiva, and cornea, respectively. Assay performance was monitored by the quality control samples.
Pharmacokinetic Analysis
The maximum concentration (Cmax), the time to reach the Cmax (Tmax), and the values of the area under the curve from 0 to 24 h after administration (AUC0–24) were calculated with Phoenix WinNonlin Build 8.1.0 software (Certara L.P., Princeton, NJ, USA) using non-compartmental analysis as the sparse sampling method.
Results
Azithromycin was rapidly absorbed into the extraocular tissues after administration (Table 1; Fig. 1). The azithromycin concentrations in the examined tissues were sustained at nearly Cmax levels for 24 h after dose administration; i.e., at 24 h post-administration, the concentrations in the eyelid, conjunctiva, and cornea were 50.6%, 84.8%, and 42.2% of their respective Cmax value. Levofloxacin was rapidly absorbed into the extraocular tissues, but its concentration decreased with time after reaching the Cmax. At 24 h post-administration, its concentrations in the eyelid, conjunctiva, and cornea were 5.0%, 0.5%, and 0.1% of their respective Cmax value. Ofloxacin was also rapidly absorbed into the extraocular tissues, and its concentrations decreased with time after the Cmax. At 24 h after the ofloxacin administration, its concentrations in the eyelid, conjunctiva, and cornea were 16.6%, 2.7%, and 1.7% of their respective Cmax value.
Discussion
Azithromycin has a long elimination half-life in the ocular tissues after ophthalmic instillation [9, 10]. Similar to previous reports, our study showed that azithromycin concentrations rapidly reached the Cmax in the extraocular tissues, which were sustained at high levels until 24 h after instilling 1% azithromycin ophthalmic solution. In contrast, we found that after instilling the ophthalmic formulations of levofloxacin or ofloxacin, their concentrations rapidly reached the Cmax in the conjunctiva and cornea, and then decreased with time. In addition, we report for the first time that the concentrations of these drugs in the eyelid also rapidly reached the Cmax and decreased with time. Thus, our results indicate that after ocular administration, the elimination profile of azithromycin in any extraocular tissue differs from that of levofloxacin or ofloxacin. Azithromycin showed high retention in cells due to its weakly basic nature, which likely led to its prolonged terminal half-life in the body [11]. These characteristics of azithromycin may lead to its slow elimination in the ocular tissues, compared with that of the other two drugs.
Adding polycarbophil to an ophthalmic formulation ensures a high bioavailability of the active pharmaceutical ingredient in the ocular tissues by retaining it on the ocular surface after ophthalmic instillation [9]. We found that the Cmax value of azithromycin in any tissue was comparable to or higher than that of levofloxacin and ofloxacin. Moreover, the AUC0–24 values of azithromycin in all tissues were higher, and the differences in the AUC0–24 values were larger than those in the Cmax values. These findings indicate that the high drug exposure in the extraocular tissues observed after the topical instillation of 1% azithromycin ophthalmic solution containing polycarbophil was dependent on both the characteristic azithromycin elimination profile and polycarbophil.
The structural properties of ofloxacin and levofloxacin are extremely similar, as ofloxacin is the racemic mixture of levofloxacin [12,13,14]. In rabbits, instilling ophthalmic solutions of levofloxacin and ofloxacin resulted in similar drug concentration values and comparable profiles in the cornea [15]. Interestingly, in our study, the pharmacokinetic properties of the 1.5% levofloxacin ophthalmic solution and the 0.3% ofloxacin ophthalmic ointment varied notably in the extraocular tissues. Our data showed that the Cmax of ofloxacin in the cornea was eightfold lower than that of levofloxacin. In the conjunctiva, however, the Cmax of ofloxacin was twofold lower than that of levofloxacin, although there was an approximately fourfold difference in the dosing amount of active pharmaceutical ingredient between these drugs. Furthermore, the ofloxacin Cmax value in the eyelid was higher than that of levofloxacin. These results imply that an ophthalmic ointment is a dose formulation for effectively delivering drugs to the eyelid and conjunctiva with limited delivery to the cornea. Certain ointment characteristics might have contributed to the observed tissue-specific differences in the drug distributions: (1) low dispersibility from the conjunctival sac contributed to the low accessibility to the cornea and the high distribution to the eyelid and conjunctiva; (2) long-term sustainability at the ocular surface resulted in high penetration of the tissues; and (3) the hydrophobic base affected the tissue permeability. A previous study found that drug concentrations in the eyelid margin after instilling an ophthalmic solution were higher than in other parts of the eyelid [16]. We observed that a portion of ointment leaking from the conjunctival sac was frequently localized to the eyelid margin. The remarkably high Cmax in the eyelid after administering a dose of ofloxacin ophthalmic ointment might have been caused by high absorption through the eyelid margin such as through the eyelash follicles.
The antimicrobial activity of azithromycin is not as potent as that of levofloxacin or ofloxacin against a large proportion of bacteria isolated from the ocular surface [17, 18]. In our study, the topical instillation of 1% azithromycin ophthalmic solution containing polycarbophil attained the highest drug exposure in all extraocular tissue among the three drugs, including the ointment formulation. Our data might suggest that the high exposure after instillation of 1% azithromycin ophthalmic solution with polycarbophil could compensate for its moderate antimicrobial activity against the specific bacteria. Furthermore, earlier reports indicated that azithromycin accumulates in polymorphonuclear leukocytes [19, 20], and is distributed to infected tissues after oral dosing [21]. Thus, these specific pharmacokinetic behaviors might cause additional exposure of azithromycin in the infected ocular tissues and improve the antimicrobial efficacy after ophthalmic instillation. In clinical practice, the approved 1% azithromycin ophthalmic solution is indicated for treating conjunctivitis and can be used in blepharitis therapy in the United States [8].
Our study showed that the decline in levofloxacin and ofloxacin concentrations from the Cmax to the levels at 24 h post-administration was slower in the eyelid than in the conjunctiva and cornea. Unlike the conjunctiva and cornea, the eyelid has a complex structure with multiple compartments, including the muscle, skin, meibomian gland, and eyelash. We measured the drug concentrations for the whole eyelid in this study. However, a previous study observed sustained drug concentrations in the eyelid margin but not in the conjunctival epithelium or meibomian gland [16]. Thus, studying intra-tissue concentrations of levofloxacin and ofloxacin in the eyelid may help reveal the reasons for differences in inter-tissue concentrations of the antimicrobial agents observed in our study.
The current study was based on single-dose pharmacokinetic data in the extraocular tissues of rabbits. However, the respective approved indications for 1% azithromycin ophthalmic solution, 1.5% levofloxacin ophthalmic solution, and 0.3% ofloxacin ophthalmic ointment are multiple-dose and different for each drug. Further multiple-dose pharmacokinetic studies with the respective clinical usage would be needed to more precisely estimate the antimicrobial potential of these drugs.
Conclusion
Our study showed that there were differences in the drug concentration profiles in the eyelid, conjunctiva, and cornea of rabbits following ophthalmic dosing of azithromycin ophthalmic solution, levofloxacin ophthalmic solution, and ofloxacin ophthalmic ointment. Concentrations of azithromycin or levofloxacin were comparable among these extraocular tissues, whereas ofloxacin concentrations were markedly different. Azithromycin had a slow elimination profile in every extraocular tissue, which differed from the other two drugs. This specific behavior of azithromycin ensured higher drug exposures in these tissues following its ophthalmic administration, compared with those of the two ophthalmic comparator drugs.
References
Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5–17.
Azari AA, Barney NP. Conjunctivitis. JAMA. 2013;310(16):1721–9.
McCulley JP, Dougherty JM, Deneau DG. Classification of chronic blepharitis. Ophthalmology. 1982;89(10):1173–80.
Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database Syst Rev. 2012;5:CD005556.
Lin A, Rhee MK, Akpek EK, et al. Bacterial Keratitis Preferred Practice Pattern®. Ophthalmology. 2019;126(1):1–55.
Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126(1):56–93.
Varu DM, Rhee MK, Akpek EK, et al. Conjunctivitis Preferred Practice Pattern®. Ophthalmology. 2019;126(1):94–169.
Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(2 Suppl):S1–14.
Akpek EK, Vittitow J, Verhoeven RS, et al. Ocular surface distribution and pharmacokinetics of a novel ophthalmic 1% azithromycin formulation. J Ocul Pharmacol Ther. 2009;25(5):433–40.
Stewart WC, Crean CS, Zink RC, Brubaker K, Haque RM, Hwang DG. Pharmacokinetics of azithromycin and moxifloxacin in human conjunctiva and aqueous humor during and after the approved dosing regimens. Am J Ophthalmol. 2010;150(5):744-751.e2.
Bosnar M, Kelnerić Ž, Munić V, Eraković V, Parnham MJ. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob Agents Chemother. 2005;49(6):2372–7.
Hayakawa I, Atarashi S, Yokohama S, Imamura M, Sakano K, Furukawa M. Synthesis and antibacterial activities of optically active ofloxacin. Antimicrob Agents Chemother. 1986;29(1):163–4.
Imamura M, Shibamura S, Hayakawa I, Osada Y. Inhibition of DNA gyrase by optically active ofloxacin. Antimicrob Agents Chemother. 1987;31(2):325–7.
Une T, Fujimoto T, Sato K, Osada Y. In vitro activity of DR-3355, an optically active ofloxacin. Antimicrob Agents Chemother. 1988;32(9):1336–40.
Fukuda M, Sasaki H. Calculation of AQCmax: comparison of five ophthalmic fluoroquinolone solutions. Curr Med Res Opin. 2008;24(12):3479–86.
Asano N, Ueda K, Kawazu K. Penetration Route of the selective glucocorticoid receptor agonist sa22465 and betamethasone into rabbit meibomian gland based on pharmacokinetics and autoradiography. Drug Metab Dispos. 2017;45(7):826–33.
Asbell PA, Pandit RT, Sanfilippo CM. Antibiotic resistance rates by geographic region among ocular pathogens collected during the armor surveillance study. Ophthalmol Ther. 2018;7(2):417–29.
Haas W, Gearinger LS, Usner DW, DeCory HH, Morris TW. Integrated analysis of three bacterial conjunctivitis trials of besifloxacin ophthalmic suspension, 0.6%: etiology of bacterial conjunctivitis and antibacterial susceptibility profile. Clin Ophthalmol. 2011;5:1369–79.
Gladue RP, Bright GM, Isaacson RE, Newborg MF. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989;33(3):277–82.
Fietta A, Merlini C, Grassi GG. Requirements for intracellular accumulation and release of clarithromycin and azithromycin by human phagocytes. J Chemother. 1997;9(1):23–31.
Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(suppl A):73–82.
Acknowledgements
Funding
This research and Rapid Service Fees were supported by Senju Pharmaceutical Co. All authors had full access to the data included in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the entire work, and have given their approval for this version to be published.
Disclosures
Tatsuya Sakai is an employee of Senju Pharmaceutical Co. Keisuke Shinno is an employee of Senju Pharmaceutical Co. Masaaki Kurata is an employee of Senju Pharmaceutical Co. Akio Kawamura is an employee of Senju Pharmaceutical Co.
Compliance with Ethics Guidelines
All institutional and national guidelines for the care and use of laboratory animals were followed. The study protocol, including all experimental procedures, was approved by the Institutional Animal Care and Use Committee of Senju Pharmaceutical Co.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8983349.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License ( http://creativecommons.org/licenses/by-nc/4.0/ ), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sakai, T., Shinno, K., Kurata, M. et al. Pharmacokinetics of Azithromycin, Levofloxacin, and Ofloxacin in Rabbit Extraocular Tissues After Ophthalmic Administration. Ophthalmol Ther 8, 511–517 (2019). https://doi.org/10.1007/s40123-019-00205-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-019-00205-0