Abstract
Background
To evaluate if conjunctival epithelial cells’ expression of HLA-DR and ICAM-1 could be helpful as early topical markers of inflammation in Graves’ orbitopathy (GO).
Methods
The ocular examination evaluated a clinical activity score (CAS) by assessment of clinical features, (e.g., eyelid or conjunctival inflammation, lid width, lid closure, proptosis, ocular motility). Conjunctival epithelial cell specimens for flow-cytometric evaluations of ICAM-I and HLADR expression were collected by impression cytology from ten eyes with active GO (CAS ≥ 4 and duration ≤ 12 months), from 15 eyes with Graves’ disease (GD) without active GO (CAS 0–2) and from 15 normal specimens without any ocular disorders.
Results
The percentage of HLA-DR + conjunctival epithelial cells was significantly elevated in patients with active GO comparing to GD without active GO and healthy controls, 10.7 % (8.5–17.7) and 7.78 % (3.92–10.1) (p < 0.05) vs. control 4.89 % (3.5–5.5) (p < 0.005), respectively. The expression of ICAM − 1+ conjunctival epithelial cells was greater only in patients with GO vs. controls, 5.5 % (4.8–7.03) and 1.46 % (0.69–2.51) (p < 0.005), respectively.
Conclusion
The percentage of HLA-DR+ and ICAM-1+ conjunctival epithelial cells in patients with the active GO may serve as a topical inflammation marker in Graves’ orbitopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Development of flow cytometric analysis of cytologic specimens provides a new, sensitive and objective tool for exploring conjunctival pathology [1]. Conjunctival epithelium expression of HLA-DR (human leukocyte antigen-DR) and ICAM-1 (intercellular adhesion molecule-1) have been shown to be correlated with some topical inflammatory processes like allergic conjunctivitis, pterygium, or keratoconjunctivitis sicca (KCS) in cystic fibrosis [2–6].
During inflammation, the expression of endothelial and epithelial ICAM-1 increases [7]. ICAM-1 attracts leukocytes to the conjunctival surface and holds them there [8]. Conjunctival expression of HLA-DR is normally restricted to resident dendritic cells, but induction of class II antigens in epithelial cells has consistently been shown to be associated with conjunctival inflammatory reactions [5, 7, 8]. Graves’ orbitopathy (GO) is known to be an autoimmune process leading to inflammation of the orbital tissue [9]. In addition, almost all patients with GO show signs of ocular surface damage [10]. Hence, detection of HLA-DR and ICAM-1 molecules on the conjunctiva may suggest activity of the immunopathological process underlying Graves' orbitopathy which should be considered as an indication of intensive systemic immunosuppression.
The objective of this study was to evaluate the expression of ICAM-1 and HLA-DR in conjunctival epithelial cells by flow cytometry in patients with active GO and Graves disease (GD) without active GO, in order to estimate if the conjunctival expression of these molecules could serve as topical markers of ongoing inflammation in the active GO.
Materials and methods
Patients and controls
Conjunctival impression cytology was obtained from ten eyes (ten patients) (eight females, two males) a mean age of 35.9 ± 9.9 years (range 26–46 years) with active GO (CAS ≥ 4), from 15 eyes (15 patients)(12 females, three males) with a mean age of 36.5 ± 11.5 years (range 25–48 years) with GD without signs of active GO, recruited from the Department of Endocrinology, Diabetology and Internal Diseases of Medical University of Bialystok. Fifteen impression cytology specimens were obtained from 15 eyes of ten healthy volunteers (eight females, two males) a mean age of 34.5 ± 12 years (range 22.5–47 years) to serve as a control.
The study was carried out with approval from the Ethics Committee of the Medical University of Bialystok, in accordance with the guidelines of the Helsinki Declaration. All patients and control persons gave their informed consent prior to inclusion in this study.
Clinical examination
The ocular examination evaluated the best corrected visual acuity (Snellen charts), colour vision (Ishihara charts), intraocular pressure (applanation tonometry) and exophthalmus. The GO clinical activity score included measurements of lid aperture, marginal reflex distance, the presence of eyelid retraction, eyelid or conjunctival inflammation, biomicroscopic examination of the anterior segment, indirect ophthalmoscopy, and ocular motility.
Grouping of patients
Signs of GO in the eye were classified according to the Clinical Activity Score (CAS) classification and NOSPECS amended by EUGOGO [11]:
-
1)
active GO – CAS equal to or greater than 4 and/or NOSPECS equal to or greater than 5 (marked symptoms of GO); Manifestation of GO ≤ 12 months.
-
2)
GD without signs of active GO – CAS less than 4 and NOSPECS less than 5 (mild symptoms of GO) or no signs of ophthalmopathy.
Exclusion criteria
Patients with allergic conjunctivitis, dry eye, blepharitis, uveitis, infective conjunctivitis or keratitis were excluded from the study. Also, patients with a history of ocular surgery within the last three months or patients with any other ocular disease that might alter the markers to be measured were excluded from the study. Patients receiving topical corticosteroids or anti-inflammatory treatment within the last month or patients who had radioiodine therapy or with any immune system disease were also excluded.
Impression cytology of conjunctival epithelial cells and flow cytometric assessment
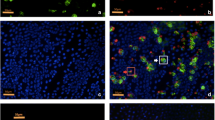
After topical anaesthesia with 1–2 drops of 0.04 % oxybuprocaine, two parts 13 × 6.5 mm in size (polyethersulfone filter, 0.20-μm pores, Supor, Gelman Sciences, Ann Arbor, MI) were applied onto the superior and superiortemporal bulbar conjunctiva without exerting any pressure. All membranes were immediately dipped into tubes containing 1.5 ml of cold phosphate-buffered saline (PBS) with fixative (0.05 % paraformaldehyde). Cells were extracted by gentle agitation for 20 min and centrifuged (1,800 rpm, 5 min). For each sample 2,000–5,000 cells were analyzed and their fluorescence output was displayed on a log scale. Monoclonal antibodies with fluorochromes: CD54-FITC (mouse IgG1, clone 6.5B5) and antibodies against class II antigen HLA-DR-FITC and EpCAM-PerCP-Cy5.5 (mouse IgG1, clone EBA-1) antibodies were purchased from DakoCytomation (Copenhagen, Denmark) and Becton Dickinson (Mountain View, CA, USA), respectively. A nonimmune mouse IgG1-FITC (clone DAK-G01) was used as a negative isotypic control (DakoCytomation). Direct immunostainings were performed with monoclonal antibodies for 30 min at 4 º C. A two parameter analysis was performed to determine the expression of ICAM-1 and HLA-DR molecules on EpCAM positive cells (conjunctival epithelial cells). The flow cytometric analysis was performed with a Coulter Epics-XL flow cytometer (Beckman Coulter Corporation, Miami, FL, USA). The results are expressed as a percentage of positive cells (Fig. 1).
Statistical analysis
The patients'characteristic data and ocular assessments are given as mean ± SD or median with range (in brackets). The median and quartile values of the ICAM-1+ and HLADR+ conjunctival epithelial cells were presented. The statistical significance of the evaluated CEC subsets’ expression was measured by the U Mann–Whitney test. A p value of less than 0.05 was considered statistically significant. All data were performed using Statistica 9.0 (StatSoft ®, Tulsa, OK, U.S.A.).
Results
Clinical features of the study groups
All results obtained for GO dependent alterations have been summerized in Table 1.
The expression of HLA-DR and ICAM-1 on CECs
The percentage of HLA-DR+ conjunctival epithelial cells was significantly elevated in patients with active GO comparing to GD without active GO and versus healthy controls (p < 0.005 and p < 0.,05 respectively) (Table 2, Fig. 2). Moreover the median percentage of HLA-DR+ CEC in active GO (10.7 % (8.5–17.7) was significantly greater than in patients with GD without active GO (7.78 % (3.92–10.1) (p < 0.05)). The median percentage of HLA-DR + epithelial cells in active GO was two-folds higher than in healthy controls (10.7 % (8.5–17.7) vs. 4.89 % (3.5–5.5)) (Table 2, Fig. 2).
The median and the interquartile range of (a) HLA-DR+, and (b) ICAM-1+ conjunctival epithelial cells’ percentage in patient with active GO, GD without active GO and controls (whiskers display the interquartile range). (c) scatter plots of HLA-DR + CEC, and (d) scatter plots of ICAM-1+ CEC in patients with active GO, GD without active GO and controls (bold line displays median value)
The statistical analysis of ICAM-1+ conjunctival epithelial cells revealed the elevated percentage of these cells in patients with active GO vs. controls (5.5 % (4.8–7.03) and 1.46 % (0.69–2.51), respectively)(p < 0.005) (Table 2, Fig. 2). In patients with GD without active GO the expression of ICAM-1+ CECs was not increased comparing to control (2.42 % (0.94–7.05) (Table 2).
Discussion
It was shown that incomplete blink, increased proptosis and greater palpebral fissure width in GO accelerates tear evaporation, which increases the fluid’s osmolarity and results in ocular surface damage [10]. Recently, it has been found that inflammation plays a key role in ocular surface damage of GO [12–15]. The increased concentrations of tears’ inflammatory cytokines (IL-1β, IL- 6) and chemokine IL-8 in patients with active vs. inactive TAO (thyroid associated ophthalmopathy) supports this thesis [16]. Gupta suggested that TAO was a potential (occult) cause of inflammatory ocular surface disease with dry eye symptoms [12].
In our previous study, serum L-selectin and ICAM-1 were found to be elevated in patients with active GO, suggesting enhanced T cell recruitment [17]. In accordance with that, we have shown an increased percentage of L-selectin + and ICAM-1+ peripheral blood CD4+/CD8+ T cells in active GO [18]. Since the orbital tissues are infiltrated with inflammatory and immune cells in GO [10], the ICAM-1 and the HLA-DR expression on the ocular surface may serve as markers of a inflammatory status in GO. ICAM-1 plays a central role not only in T lymphocyte activation but also T cell trafficking at the inflammatory sites, which may result in amplification of the cellular immune process in active GO (Bahn 2003). The presence of HLA-DR renders conjunctival epithelial cells capable of presenting antigens, as investigated by impression cytology, which could be an indirect indicator of the involvement of the Th1 subset [19]. Not only the increased percentage of HLA-DR+ CECs in patients with active GO vs. controls, but also vs. GD patients without active GO may suggests the local activation in these patients is due to different immunological pathways. It has been shown that the Th1 profile predominates in the early active phase of GO [20], which can be attributed to the local HLA-DR engagement. In agreement with that, our study showed a significantly higher percentage of HLA-DR + CECs in patients with active GO than in patients without active GO.
Kulig et al., found that the serum levels of ICAM-1 seem to be a more sensitive marker than MRI in the assessment of the activity of GO [21]. Our study is one of the few that show the topical activation markers may be useful for diagnosing active GO. Further studies should be designed to elucidate the role of conjunctival epithelial cells’ immunophenotyping in monitoring the effects of systemic immunomodulatory treatment in active GO [17].
In conclusion, our results might be a good hint that the HLA-DR + CECs and ICAM-1+ CECs immunophenotyping could be a topical inflammation marker in active GO.
References
Baudouin C, Brignole F, Becquet F, Pisella PJ, Goguel A (1997) Flow cytometry in impression cytology specimens. A new method for evaluation of conjunctival inflammation. Invest Ophthalmol Vis Sci 38:1458–1464
Mrugacz M, Zak J, Bakunowicz-Lazarczyk A, Wysocka J, Kaczmarski M (2007) ICAM-1 expression on conjunctival epithelial cells in patients with cystic fibrosis. Cytometry B Clin Cytom 72:204–208
Mrugacz M, Zak J, Bakunowicz-Lazarczyk A, Wysocka J, Minarowska A (2007) Flow cytometric analysis of HLA-DR antigen in conjunctival epithelial cells of patients with cystic fibrosis. Eye (Lond) 21:1062–1066
Okada N, Fukagawa K, Takano Y, Dogru M, Tsubota K, Fujishima H, Matsumoto K, Nakajima T, Saito H (2005) The implications of the upregulation of ICAM-1/VCAM-1 expression of corneal fibroblasts on the pathogenesis of allergic keratopathy. Invest Ophthalmol Vis Sci 46:4512–4518
Tekelioglu Y, Turk A, Avunduk AM, Yulug E (2006) Flow cytometrical analysis of adhesion molecules, T-lymphocyte subpopulations and inflammatory markers in pterygium. Ophthalmologica 220:372–378
Tsubota K, Fujihara T, Saito K, Takeuchi T (1999) Conjunctival epithelium expression of HLA-DR in dry eye patients. Ophthalmologica 213:16–19
Gao J, Morgan G, Tieu D, Schwalb TA, Luo JY, Wheeler LA, Stern ME (2004) ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjogrens syndrome-like MRL/lpr mice. Exp Eye Res 78:823–835
Hingorani M, Metz D, Lightman SL (1997) Characterisation of the normal conjunctival leukocyte population. Exp Eye Res 64:905–912
Lutt JR, Lim LL, Phal PM, Rosenbaum JT (2008) Orbital inflammatory disease. Semin Arthritis Rheum 37:207–222
Eckstein AK, Johnson KT, Thanos M, Esser J, Ludgate M (2009) Current insights into the pathogenesis of Graves' orbitopathy. Horm Metab Res 41:456–464
Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits MP, Perros P, Boboridis K, Boschi A, Curro N, Daumerie C, Kahaly GJ, Krassas G, Lane CM, Lazarus JH, Marino M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM (2008) Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid 18:333–346
Gupta A, Sadeghi PB, Akpek EK (2009) Occult thyroid eye disease in patients presenting with dry eye symptoms. Am J Ophthalmol 147:919–923
Versura P, Campos EC (2010) The ocular surface in thyroid diseases. Curr Opin Allergy Clin Immunol 10:486–492
Villani E, Galimberti D, Viola F, Ratiglia R (2010) In vivo confocal microscopy of the ocular surface. Am J Ophthalmol 149:689–690
Yoon JS, Choi SH, Lee JH, Lee SJ, Lee SY (2010) Ocular surface inflammation, and nerve growth factor level in tears in active thyroid-associated ophthalmopathy. Graefes Arch Clin Exp Ophthalmol 248:271–276
Huang D, Xu N, Song Y, Wang P, Yang H (2012) Inflammatory cytokine profiles in the tears of thyroid-associated ophthalmopathy. Graefes Arch Clin Exp Ophthalmol 250:619–625
Mysliwiec J, Kretowski A, Szelachowska M, Topolska J, Mikita A, Kinalska I (2001) Serum L-selectin and ICAM-1 in patients with Graves' ophthalmopathy during treatment with corticosteroids. Immunol Lett 78:123–126
Pawlowski P, Mysliwiec J, Stasiak-Barmuta A, Bakunowicz-Lazarczyk A, Gorska M (2009) Increased percentage of L-selectin + and ICAM-1+ peripheral blood CD4+/CD8+ T cells in active Graves' ophthalmopathy. Folia Histochem Cytobiol 47:29–33
Baudouin C, Bourcier T, Brignole F, Bertel F, Moldovan M, Goldschild M, Goguel A (2000) Correlation between tear IgE levels and HLA-DR expression by conjunctival cells in allergic and nonallergic chronic conjunctivitis. Graefes Arch Clin Exp Ophthalmol 238:900–904
Bahn RS (2003) Clinical review 157: Pathophysiology of Graves' ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab 88:1939–1946
Kulig G, Pilarska K, Kulig J, Krzyzanowska-Swiniarska B, Andrysiak-Mamos E, Robaczyk M (2004) Magnetic resonance imaging and soluble forms of adhesion molecules: sICAM and sVCAM in assessing the activity of thyroid orbitopathy. Pol Arch Med Wewn 111:161–169
Acknowledgments
This work was supported by the research grant of the Medical University of Bialystok. The preliminary results of this study were presented and awarded by the scientific section of immunology/microbiology of European Vision and Eye Research (EVER) during the EVER 2011 Congress in Crete, Greece 5–8 October 2011.
Financial disclosures
None.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pawlowski, P., Mysliwiec, J., Mrugacz, M. et al. Elevated percentage of HLA-DR+ and ICAM-1+ conjunctival epithelial cells in active Graves’ orbitopathy. Graefes Arch Clin Exp Ophthalmol 252, 641–645 (2014). https://doi.org/10.1007/s00417-014-2580-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2580-z