Abstract
Background
Status epilepticus (SE) is a heterogeneous neurological emergency with significant variability in prognosis, influenced by underlying disease and pathophysiological context. Acid–base disturbances are common in critically ill patients, yet their distribution and impact in SE patients remain poorly understood.
Methods
This was an observational cohort study including non-hypoxic SE patients with available blood gas analysis within the first 24 h of SE, treated at the University Hospital of Geneva, Switzerland between 2015 and 2023. Acid–base disturbances were classified using the Henderson–Hasselbalch equation, with prevalent metabolic alkalosis confirmed through the Stewart approach. Primary outcomes were in-hospital mortality, Glasgow Outcome Scale (GOS) at discharge, and return to premorbid neurologic function.
Findings
Among 540 SE patients, 365 were included. Half of patients exhibited acid–base disturbances within the initial 24 h of SE, with metabolic and respiratory acidosis being the most prevalent, though not prognostically significant. After correction for possible confounders, metabolic alkalosis (6%) was associated with increased in-hospital mortality (P = 0.011; OR = 4.87, 95% CI = 1.29–7.84), worse GOS (P = 0.012; OR = 3.18, 95% CI = 1.29–7.84), and reduced likelihood of returning to premorbid function (P = 0.017; OR = 3.30, CI95% = 1.24–8.80). Following the Stewart approach, 9% of patients had predominant metabolic alkalosis, associated with worse GOS (P = 0.005; OR:3.37, 95%CI = 1.45–7.82), and reduced chance of returning to baseline (P = 0.012; OR = 3.29, CI95% = 1.30–8.32). Metabolic alkalosis was related to hypoalbuminemia and lower serum potassium.
Conclusion
Metabolic alkalosis strongly predicts mortality and adverse functional outcome in SE patients. Prospective studies should assess whether early detection and correction of metabolic alkalosis and related electrolyte imbalances can improve SE prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Status epilepticus (SE) is a neurological emergency with ongoing epileptic seizures [1]. This condition is highly heterogeneous, and it is often described as a complication of other acute or chronic insults rather than a disease itself [2].
Prognosis in SE is highly variable, ranging from severe neurological disability to death [1, 3, 4]. The outcome of SE is determined by the underlying disease but is also heavily influenced by the pathophysiological context in which it develops, including both factors independent from SE (such as age, comorbidities, concomitant therapy) and/or triggered by SE (such as organ, metabolic, and electrolytic dysfunctions) [5]. This bidirectional relationship makes prognostication particularly challenging [2, 6].
It has been demonstrated that low brain and blood pH levels strongly suppress epileptic activity [7, 8], while emerging evidence suggests that alkalosis may trigger febrile and hypoxic-related seizures [9, 10]. However, few studies have systematically examined acid–base disorders and their association with outcome in SE patients [11].
Notably, pH alterations are common in critically ill patients and can substantially impact patients’ outcome [12]. SE may cause multiple acid–base disturbances, including respiratory acidosis due to impaired respiratory function and metabolic acidosis from anaerobic muscle metabolism [11, 13, 14].
Our study aims to investigate acid–base disturbances in a large and heterogeneous SE population, examining the relationship between acid–base disorders, functional outcomes, and mortality.
Methods
Data collection and definitions
This is an observational cohort study performed at the University Hospital of Geneva (HUG), a Swiss academic tertiary medical care center. The STROBE guidelines were followed to improve the quality of the study [15].
Data from all adult patients (aged ≥ 18 years) treated for SE between November 1st, 2015 and December 31st, 2023 were retrospectively identified from a SE registry retrospectively collected to October 2021, and prospectively collected from November 2021 to 2023.
Data were collected and managed with the password encrypted online browser-based, metadata-driven database organizer REDCAP (Research Electronic Data Capture) [16].
Patients with SE following cardiorespiratory arrest and patients without blood gas analysis (BGA) during the first 24 h of SE were excluded.
The following features were retrieved: patients’ age, sex, comorbidities, SE etiology, semiology, and duration. SE types were defined as recommended by the International League Against Epilepsy (ILAE) [1]. SE etiology was defined as acute symptomatic, remote symptomatic, progressive symptomatic, and unknown [1]. SE etiology was categorized as potential non-fatal and fatal, following previous reports [17].
Charlson Comorbidity Index (CCI, range 0–37) and the Status Epilepticus Severity Score (STESS, range 0–6) were calculated to quantify comorbidity burden and illness severity, respectively [18,19,20].
Sepsis has been defined in the presence of established infection together with organ dysfunction, represented by an increase in the Sequential (sepsis-related) Organ Failure Assessment (SOFA) score of 2 points or more [21, 22].
SE duration was defined as the period between SE diagnosis and the clinical and/or EEG evidence of seizure termination, as described elsewhere [23].
The Glasgow Outcome Scale (GOS, range 1–5) was used to assess outcome at discharge, dichotomized as follows: bad outcome—GOS of 1 to 3; good outcome—GOS of 4 to 5 [24].
Values of pH, partial pressure of carbon dioxide (PaCO2) and bicarbonate (HCO3–) from BGA during the first 24 h of SE were gathered. The following laboratory data within the first 24 h of SE were also collected: serum albumin [g/L], serum creatinine [umol/L], serum phosphate [umol/L], serum bilirubin [umol/L], serum urea [umol/L], serum sodium [mmol/L], and serum potassium [mmol/L].
Values obtained beyond 24 h from SE onset were excluded. For patients with multiple laboratory values within the first 24 h, only the first assessment was considered.
According to the Henderson–Hasselbalch equation [25], the following acid–base disturbance categories were defined: acidosis (either Respiratory [pH < 7.35, PaCo2 > 45 mmHg], metabolic [pH < 7.35, HCO3 < 22 mmol/L] or mixt [pH < 7.35, PaCo2 > 45 mmHg, HCO3 < 22 mmol/L]), normal pH (pH 7.35–7.45) and alkalosis (either respiratory [pH > 7.45, PaCo2 < 35 mmHg], metabolic [pH > 7.45, HCO3 > 26 mmol/L] or mixt [pH > 7.45, PaCo2 < 35 mmHg, HCO3 > 26 mmol/L])[26, 27].
In addition, to better identify predominant metabolic alkalosis in patients with complex acid–base disorders, we used the Stewart approach, which posits that the body’s pH is primarily determined by the difference in charge between strong ions and weak acids. According to this method, the presence of metabolic alkalosis is indicated by an effective Strong Ion Difference (SIDe) > 40 mEq/L, and/or serum hypoalbuminemia (< 35 g/L). We defined metabolic alkalosis as the predominant acid–base disturbances in the presence of pH > 7.45, SID > 40 mE/L and/or hypoalbuminemia [28, 29].
Outcomes
Primary outcomes were the relationships among acid–base categories and in-hospital mortality, GOS at discharge [24] and return to baseline premorbid neurologic function at discharge.
Secondary outcome was the distribution of acid–base disturbances according to the Henderson–Hasselbalch equation in the SE patients.
Statistics
Univariable comparisons were performed by the χ2 test for categorical variables. For continuous variables, the Shapiro–Wilk test was used to distinguish between normally and not normally distributed variables.
We assessed relationship among acid–base disturbances according to the Henderson–Hasselbalch equation and primary outcomes first through univariate analyses and then through a binomial regression model considering unbalanced univariate results and variables potentially related to outcomes (age, CCI, SE semeiology, SE potentially fatal etiology, SE duration, STESS, and reliance of invasive therapies).
Then we examined the association between predominant metabolic alkalosis according to the Stewart approach and primary outcomes using both univariate analyses and a binomial regression model.
We assessed laboratory variables associated with the presence of metabolic alkalosis according to according to the Henderson–Hasselbalch equation and the Stewart approach through univariate analysis.
Finally, given the known correlation between sepsis and acid–base unbalance, we performed a subgroup analysis considering only patients with sepsis and evaluating acid–base distribution in this population together with correlation with outcome.
All analyses were performed utilizing the Jamovi software (2.3.21.0 Version).
Results
Baseline population features
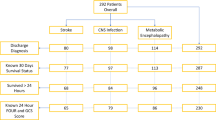
From 540 identified patients treated for SE during the study period, 44 patients suffered SE after cardiac arrest and were excluded from analysis. Of the remaining 496 patients, BGA during the first 24 h of SE was available in 365 patients, included in the study. Of them, 34 (9%) died during hospital stay, 113 (31%) were discharged with a GOS of 1 to 3, and 160 (44%) did not return to baseline premorbid neurologic function.
Baseline and SE features associated with primary outcomes at univariate analysis are presented in Table 1. Overall, 209 (57%) of SE patients were male, more than 50% required ICU admission, and mean age at SE onset was 61 years. A history of epilepsy was present in 163 (45%). Age and CCI were both associated with in-hospital mortality (P = 0.005 and P < 0.001, respectively) and no return to premorbid neurologic function at discharge (P < 0.001). Mean SE duration and mean STESS values were associated with in-hospital mortality (both P < 0.001), GOS 1–3 (P < 0.001 and P = 0.005, respectively), and no return to premorbid neurologic function at discharge (both P < 0.001).
SE with prominent motor symptoms was diagnosed in 248 (68%) patients, while 117 (32%) showed NCSE, which was associated with a significantly higher in-hospital mortality rate (P = 0.019).
Acid–base categories according to the Henderson–Hasselbalch equation: univariate analysis
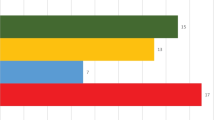
Acid–base category distribution among SE patients is graphically represented in Fig. 1. Normal mean pH values in the first 24 h of SE were found in 187 (51%) patients. Of them, 6% died during hospital stay, 28% was discharged with a GOS of 1 to 3, and 39% did not regain premorbid neurologic condition at discharge.
Of the 123 (34%) SE patients with a pH < 7.35, respiratory acidosis was found in 48 (14%) patients (in-hospital mortality: 13%; GOS 1–3 at discharge: 25%; no return to baseline neurologic function at discharge: 38%), metabolic acidosis in 59 (16%) patients (in-hospital mortality: 10%; GOS 1–3 at discharge: 28%; no return to baseline neurologic function at discharge: 42%), and mixt acidosis in 16 (4%) patients (in-hospital mortality: 13%; GOS 1–3 at discharge: 25%; no-return to baseline neurologic function at discharge: 44%).
Of the 55 (15%) SE patients with a pH > 7.45, respiratory alkalosis was found in 26 (7%) patients (in-hospital mortality: 10%; GOS 1–3 at discharge: 50%; no-return to baseline neurologic function at discharge: 61%), metabolic alkalosis in 23 (6%) patients (in-hospital mortality: 22%; GOS 1–3 at discharge: 52%; no-return to baseline neurologic function at discharge: 69%), and mixt alkalosis in 6 (2%) (in-hospital mortality: 0%; GOS 1–3 at discharge: 33%; no return to baseline neurologic function at discharge: 67%).
At univariate analysis, a normal pH value was associated to reduced in-hospital mortality (P = 0.020), while respiratory alkalosis was associated with GOS 1–3 at discharge (P = 0.030). Notably, the presence of metabolic alkalosis was associated with increased in-hospital mortality (P = 0.034), GOS 1–3 at discharge (P = 0.023), and reduced chance to return to baseline premorbid neurologic function at discharge (P = 0.010). Table 2 shows univariate analysis assessing the relationship between acid–base categories and primary outcomes.
Acid–base categories according to the Henderson–Hasselbalch equation: multivariate analysis
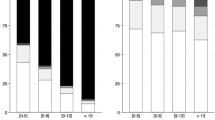
Binomial regression assessing acid–base categories together with age, CCI, SE semeiology, SE potentially fatal etiology, SE duration, STESS, and reliance of invasive therapies revealed that metabolic alkalosis was independently associated with increased risk of in-hospital mortality (P = 0.011; odds ratio [OR]:4.87, 95% confidence interval [CI] 1.29–7.84), GOS 1–3 at discharge (P = 0.012; OR: 3.18, 95% CI 1.29–7.84), and reduced chance to return to baseline neurologic function (P = 0.017; OR: 3.30, CI95% 1.24–8.80). Conversely, all other categories were not related to primary outcomes after correction for other prognostic-related variables. Multivariate analysis results are highlighted in Fig. 2.
Binomial regression analysis assessing relationship among acid–base categories following the Henderson–Hasselbalch approach and primary outcomes, after adjusting for confounding factors (age, CCI, SE semeiology, SE potentially fatal etiology, SE duration, STESS, and reliance of invasives therapies). OR odds ratio, CI confidence interval, GOS Glasgow Outcome Scale. Bold fond indicates statistical significance
Predominant metabolic alkalosis according to the Stewart equation: univariate and multivariate analyses
Data were available for 315 patients. Of them, 116 (37%) presented a SIDe > 40 mEq/L and/or hypalbuminemia. Predominant metabolic alkalosis as previously defined was present in 30 (9%) patients and was associated to in-hospital mortality (P = 0.040), GOS 1–3 at discharge (P = 0.001), and an augmented likelihood of not returning to premorbid neurologic function (P = 0.005) at univariate analysis. After correction for potential confounders, predominant metabolic alkalosis was still related to worse GOS at discharge (P = 0.005; OR: 3.37, 95% CI 1.45–7.82), and reduced chance of returning to baseline (P = 0.012; OR: 3.29, CI95% 1.30–8.32).
Exploratory analyses: laboratory values associated with metabolic alkalosis
Patients with metabolic alkalosis following the Henderson–Hasselbalch equation exhibited significantly lower serum albumin levels (34.0 [± 5.8] g/L vs 39 [± 7.0] g/L; P = 0.001) and lower serum potassium levels (3.4 [± 0.4] mmol/L vs 3.9 [± 0.6] mmol/L; P < 0.001) compared to other patients, while no significant differences were observed in serum creatinine, serum bilirubin, serum urea, or serum sodium levels.
Patients with predominant metabolic alkalosis following the Stewart equation exhibited significantly lower serum albumin levels (32.0 [± 4.7] g/L vs 40 [± 6.1] g/L; P < 0.001), as a direct consequence of its definition, but also lower serum potassium levels (3.5 [± 0.5] mmol/L vs 3.9 [± 0.6] mmol/L; P = 0.007) compared to other patients, while no significant differences were observed in serum creatinine, serum bilirubin, serum urea, or serum sodium levels.
Subgroup analysis: septic patients
Mean pH of the 43 (12%) patients with sepsis was 7.37 [± 0.10]. Eleven (26%) patients showed acidosis (four respiratory, six metabolic, one mixt acidosis), six (14%) patients showed alkalosis (five respiratory, one metabolic), and twenty-six (60%) showed a pH within the normal range. Considering the Stewart approach, five patients (12%) presented a prevalent metabolic alkalosis, significantly related to in-hospital death (P = 0.040).
Discussion
Our study reveals that approximately 50% of SE patients present with an acid–base disorder within the first 24 h of SE according to the Henderson–Hasselbalch approach.
About 35% of patients experienced respiratory and/or metabolic acidosis, likely directly related to respiratory disfunction and/or lactate production [11, 13, 14]. The antiepileptic effect of acidosis is well established: neuronal excitability and seizure activity are strongly suppressed by a decrease in brain and blood pH [7, 8], with some anticonvulsants, such as acetazolamide, acting reducing extracellular brain pH [30]. However, the prognostic role of acidosis in critically ill patients remains debated. Some studies associate acidosis to higher mortality [31, 32], while others find no relationship with outcome [33], or even a correlation to better prognosis [34, 35]. In our study, we did not find a clear association between acidosis and outcomes, but a larger sample size may be needed to detect small differences.
In the first 24 h of SE, 15% of patients showed alkalosis, split evenly between metabolic and respiratory causes. The pathogenesis of alkalosis in SE is less clear, and likely influenced by the overall pathophysiological context. Alkalosis, especially metabolic alkalosis, has been recognized as a common acid–base disturbance in critically ill patients [36, 37], increasing with invasive therapies like mechanical ventilation, gastric aspiration, and intravenous infusions [38]. In our cohort, we assessed pH only in the very first stage of SE and only a small percentage of patients experienced alkalosis, with no relationship to intensive care unit (ICU) admission or invasive therapies, suggesting their non-primary role in this context.
An increase in pH is associated with the generation of spontaneous interictal spikes in animal models, potentially contributing to the interictal–ictal transition [39]. In this context, hyperventilation-induced alkalosis has long been used to induce seizure activity/electroencephalogram abnormalities as an activation test [40].
Several reports highlighted that the presence of metabolic alkalosis is associated with longer ICU stays and increased mortality in patients with various diseases [41,42,43]. In our cohort, metabolic alkalosis at admission was a strong indicator of higher mortality, worse GOS, and a lower likelihood of returning to baseline even after correction for potential confounders.
Our findings were further validated using the Stewart approach, which, despite being more complex and less user-friendly for clinical practice, offers a more detailed and precise assessment of acid–base disorders, particularly in critical care settings [28, 29]. According to the Stewart method, predominant metabolic alkalosis was associated with all primary outcomes in univariate analysis and remained significantly linked to a reduced likelihood of good functional outcomes after binomial regression.
Metabolic alkalosis may directly contribute to poor outcomes or be an indicator of other adverse factors. Some studies suggest that metabolic alkalosis directly impacts outcomes by reducing respiratory input, inhibiting hemoglobin dissociation and causing electrolytes imbalance [41, 44]. We analyzed the number of patients with metabolic alkalosis according to the Henderson–Hasselbalch approach who normalized their pH levels within the subsequent 24 h: of 17 patients with available data, the normalization of pH had no clear impact on prognosis, but the extremely small sample size warrants caution in interpretation.
While alkalosis may contribute directly (e.g., through respiratory depression), other factors likely play a significant role in reducing the likelihood of good outcome for alkalosis patients in our cohort. We found a significant correlation between metabolic alkalosis, hypoalbuminemia, and lower serum potassium. The association between alkalosis and hypoalbuminemia is well established [45], as well as the bidirectional relationship among metabolic alkalosis and hypokalemia [46].
Hypoalbuminemia is a well-known predictor of poor outcomes across various conditions, including cardiovascular disease, cancer, and sepsis [47,48,49,50], and it might be considered as a reliable biomarker of overall patient’s resources to survive SE. Similarly, low serum potassium has been associated with adverse outcomes in critically ill patients, including an increased risk of respiratory insufficiency, with a reduced capacity to wean from mechanical ventilation [51].
Our study has several limitations. First, as a retrospective cohort study covering nearly a decade, it may have been affected by changes in the management of SE patients over time. In addition, we did not account for pre-hospital management, which could have influenced pH levels in the early stages of SE or at hospital admission. Furthermore, we were unable to evaluate long-term outcomes due to a lack of data. Another limitation is the variability in the timing of pH assessments relative to SE onset and hospital admission, which our data do not capture. However, BGA are routinely performed during the initial assessment after hospital admission, and for patients with multiple ABG assessments, only the first one was considered.
Finally, while it is known that respiratory-induced alkalosis might facilitate seizure occurrence and respiratory-induced acidosis might elevate the seizure threshold promoting seizure cessation, our study did not directly focus on SE duration in relation to pH [8,9,10]. However, no pH categories were related to SE duration in our study, and the association between metabolic alkalosis and outcomes remained significant after adjusting for several confounders, including SE duration. This makes it very unlikely that the observed association between metabolic alkalosis and outcomes is based on SE duration.
Conclusion
In our cohort, approximately half of the patients exhibited acid–base disturbances within the first 24 h of SE, according to the Henderson–Hasselbalch approach. Although less common, metabolic alkalosis was strongly associated with in-hospital mortality and severe impairment at discharge, findings that were further confirmed using the Stewart approach.
It is unlikely that alkalosis originates from SE itself, and it remains unclear whether correcting pH values can improve outcomes. Alkalosis likely results from factors such as low serum albumin and electrolyte imbalances, which are themselves associated with poor prognosis. Robust prospective studies are needed to determine whether prompt recognition and aggressive treatment of alkalosis, hypoalbuminemia, and electrolyte imbalances can improve outcomes in SE patients.
Data sharing and availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Trinka E, Cock H, Hesdorffer D et al (2015) A definition and classification of status epilepticus: report of the ILAE Task Force on classification of status epilepticus. Epilepsia 56:1515–1523. https://doi.org/10.1111/epi.13121
Lattanzi S, Trinka E, Brigo F, Meletti S (2023) Clinical scores and clusters for prediction of outcomes in status epilepticus. Epilepsy Behav 140:109110. https://doi.org/10.1016/j.yebeh.2023.109110
Leitinger M, Trinka E, Zimmermann G et al (2020) Epidemiology of status epilepticus in adults: apples, pears, and oranges—a critical review. Epilepsy Behav 103:106720. https://doi.org/10.1016/j.yebeh.2019.106720
Misirocchi F, Zilioli A, Mannini E et al (2024) Prognostic value of Salzburg nonconvulsive status epilepticus criteria: the SACE score. Epilepsia 65:138–147. https://doi.org/10.1111/epi.17830
Sutter R, Kaplan PW, Rüegg S (2013) Outcome predictors for status epilepticus—what really counts. Nat Rev Neurol 9:525–534. https://doi.org/10.1038/nrneurol.2013.154
Yuan F, Damien C, Gaspard N (2023) Prognostic scores in status epilepticus: a systematic review and meta-analysis. Epilepsia 64:17–28. https://doi.org/10.1111/epi.17442
Jia R, Zhu G, Zhao R et al (2024) Hydrogen treatment reduces electroencephalographic activity and neuronal death in rats with refractory status epilepticus by inhibiting membrane NR2B phosphorylation and oxidative stress. J Int Med Res. https://doi.org/10.1177/03000605241235589
Ziemann AE, Schnizler MK, Albert GW et al (2008) Seizure termination by acidosis depends on ASIC1a. Nat Neurosci 11:816–822. https://doi.org/10.1038/nn.2132
Schuchmann S, Hauck S, Henning S et al (2011) Respiratory alkalosis in children with febrile seizures. Epilepsia 52:1949–1955. https://doi.org/10.1111/j.1528-1167.2011.03259.x
Helmy MM, Tolner EA, Vanhatalo S et al (2011) Brain alkalosis causes birth asphyxia seizures, suggesting therapeutic strategy. Ann Neurol 69:493–500. https://doi.org/10.1002/ana.22223
Wijdicks EFM, Hubmayr RD (1994) Acute acid-base disorders associated with status epilepticus. Mayo Clin Proc 69:1044–1046. https://doi.org/10.1016/S0025-6196(12)61370-6
Kaplan LJ, Frangos S (2004) Clinical review: acid–base abnormalities in the intensive care unit. Crit Care 9:198. https://doi.org/10.1186/cc2912
Sutter R, Dittrich T, Semmlack S et al (2018) Acute systemic complications of convulsive status epilepticus: a systematic Review. Crit Care Med 46:138–145. https://doi.org/10.1097/CCM.0000000000002843
Scholtes FB, Renier WO, Meinardi H (1994) Generalized convulsive status epilepticus: causes, therapy, and outcome in 346 patients. Epilepsia 35:1104–1112. https://doi.org/10.1111/j.1528-1157.1994.tb02562.x
von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet 370:1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Misirocchi F, De Stefano P, Zilioli A et al (2024) Periodic discharges and status epilepticus: a critical reappraisal. Clin Neurophysiol 163:124–131. https://doi.org/10.1016/j.clinph.2024.04.018
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Rossetti AO, Logroscino G, Bromfield EB (2006) A clinical score for prognosis of status epilepticus in adults. Neurology 66:1736–1738. https://doi.org/10.1212/01.wnl.0000223352.71621.97
Rossetti AO, Logroscino G, Milligan TA et al (2008) Status epilepticus severity score (STESS). J Neurol 255:1561–1566. https://doi.org/10.1007/s00415-008-0989-1
Vincent J-L, de Mendonca A, Cantraine F et al (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units. Crit Care Med 26:1793–1800. https://doi.org/10.1097/00003246-199811000-00016
Singer M, Deutschman CS, Seymour CW et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801. https://doi.org/10.1001/jama.2016.0287
De Stefano P, Baumann SM, Grzonka P et al (2023) Early timing of anesthesia in status epilepticus is associated with complete recovery: A 7-year retrospective two-center study. Epilepsia 64:1493–1506. https://doi.org/10.1111/epi.17614
Jennett B (1975) Assessment of outcome after severe brain damage a practical scale. The Lancet 305:480–484. https://doi.org/10.1016/S0140-6736(75)92830-5
Po HN, Senozan NM (2001) The Henderson–Hasselbalch equation: its history and limitations. J Chem Educ 78:1499. https://doi.org/10.1021/ed078p1499
Romanski SO (1986) Interpreting ABGs in four easy steps (continuing education credit). Nursing (Brux) 16:58–64
Larkin BG, Zimmanck RJ (2015) Interpreting arterial blood gases successfully. AORN J 102:343–357. https://doi.org/10.1016/j.aorn.2015.08.002
Quintard H, Hubert S, Ichai C (2007) Qu’apporte le modèle de Stewart à l’interprétation des troubles de l’équilibre acide–base? Ann Fr Anesth Reanim 26:423–433. https://doi.org/10.1016/j.annfar.2007.02.012
Stewart PA (1983) Modern quantitative acid–base chemistry. Can J Physiol Pharmacol 61:1444–1461. https://doi.org/10.1139/y83-207
Shukralla AA, Dolan E, Delanty N (2022) Acetazolamide: old drug, new evidence? Epilepsia Open 7:378–392. https://doi.org/10.1002/epi4.12619
Gupta AK, Zygun DA, Johnston AJ et al (2004) Extracellular brain pH and outcome following severe traumatic brain injury. J Neurotrauma 21:678–684. https://doi.org/10.1089/0897715041269722
Fujii T, Udy AA, Nichol A et al (2021) Incidence and management of metabolic acidosis with sodium bicarbonate in the ICU: an international observational study. Crit Care 25:45. https://doi.org/10.1186/s13054-020-03431-2
Corwin G, Sexton K, Beck W et al (2020) Characterization of acidosis in trauma patient. J Emerg Trauma Shock 13:213. https://doi.org/10.4103/JETS.JETS_45_19
Kapetanakis T, Siempos II, Metaxas EI et al (2011) Metabolic acidosis may be as protective as hypercapnic acidosis in an ex-vivo model of severe ventilator-induced lung injury: a pilot study. BMC Anesthesiol 11:8. https://doi.org/10.1186/1471-2253-11-8
Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER (2006) Hypercapnic acidosis and mortality in acute lung injury*. Crit Care Med 34:1–7. https://doi.org/10.1097/01.CCM.0000194533.75481.03
Gunnerson KJ (2005) Clinical review: the meaning of acid–base abnormalities in the intensive care unit—epidemiology. Crit Care 9:508. https://doi.org/10.1186/cc3796
Mæhle K, Haug B, Flaatten H, Nielsen EW (2014) Metabolic alkalosis is the most common acid–base disorder in ICU patients. Crit Care 18:420. https://doi.org/10.1186/cc13802
Tinawi M (2021) Pathophysiology, evaluation, and management of metabolic alkalosis. Cureus. https://doi.org/10.7759/cureus.12841
de Curtis M, Manfridi A, Biella G (1998) Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J Neurosci 18:7543–7551. https://doi.org/10.1523/JNEUROSCI.18-18-07543.1998
Guaranha MSB, Garzon E, Buchpiguel CA et al (2005) Hyperventilation revisited: physiological effects and efficacy on focal seizure activation in the era of video-EEG monitoring. Epilepsia 46:69–75. https://doi.org/10.1111/j.0013-9580.2005.11104.x
Libório AB, Noritomi DT, Leite TT et al (2015) Increased serum bicarbonate in critically ill patients: a retrospective analysis. Intensive Care Med 41:479–486. https://doi.org/10.1007/s00134-015-3649-9
Kreü S, Jazrawi A, Miller J et al (2017) Alkalosis in critically ill patients with severe sepsis and septic shock. PLoS ONE 12:e0168563. https://doi.org/10.1371/journal.pone.0168563
Anderson LE, Henrich WL (1987) Alkalemia-associated morbidity and mortality in medical and surgical patients. South Med J 80:729–733. https://doi.org/10.1097/00007611-198706000-00016
Webster NR, Kulkarni V (1999) Metabolic alkalosis in the critically III. Crit Rev Clin Lab Sci 36:497–510. https://doi.org/10.1080/10408369991239286
McAuliffe JJ, Lind LJ, Leith DE, Fencl V (1986) Hypoproteinemic alkalosis. Am J Med 81:86–90. https://doi.org/10.1016/0002-9343(86)90187-7
Aronson PS, Giebisch G (2011) Effects of pH on potassium. J Am Soc Nephrol 22:1981–1989. https://doi.org/10.1681/ASN.2011040414
Schalk BWM, Visser M, Bremmer MA et al (2006) Change of serum albumin and risk of cardiovascular disease and all-cause mortality. Am J Epidemiol 164:969–977. https://doi.org/10.1093/aje/kwj312
Yap FHY, Joynt GM, Buckley TA, Wong ELY (2002) Association of serum albumin concentration and mortality risk in critically ill patients. Anaesth Intensive Care 30:202–207. https://doi.org/10.1177/0310057X0203000213
Jin X, Li J, Sun L et al (2022) Prognostic value of serum albumin level in critically ill patients: observational data from large intensive care unit databases. Front Nutr. https://doi.org/10.3389/fnut.2022.770674
Kim S, McClave SA, Martindale RG et al (2017) Hypoalbuminemia and clinical outcomes: what is the mechanism behind the relationship? Am Surg. https://doi.org/10.1177/000313481708301123
Hessels L, Hoekstra M, Mijzen LJ et al (2015) The relationship between serum potassium, potassium variability and in-hospital mortality in critically ill patients and a before-after analysis on the impact of computer-assisted potassium control. Crit Care 19:4. https://doi.org/10.1186/s13054-014-0720-9
Funding
Open access funding provided by University of Geneva. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Francesco Misirocchi, Hervé Quintard, and Pia De Stefano. The first draft of the manuscript was written by Francesco Misirocchi, and Pia De Stefano. The manuscript was revised by Francesco Misirocchi, Hervé Quintard Pia De Stefano, and Margitta Seeck. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
FM is supported by the 2023 International Federation of Clinical Neurophysiology (IFCN) Research Fellowship Grant. HQ declares no competing interests. MS is a shareholder of Epilog NV (Ghent, Belgium). She received grants from the Swiss National Science Foundation (163398, CRS115- 180365). PDS was supported by the Swiss National Science Foundation (163398, CRS115-180365) and is supported by the 2022 Swiss League Against Epilepsy Research Support Prize.
Ethical standards
The study protocol was reviewed and approved by the local ethic committee (CCER 2019–00836), and patients’ consent was waived in compliance with the Declaration of Helsinki first published in 1964 and its following amendments.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Misirocchi, F., Quintard, H., Seeck, M. et al. Metabolic alkalosis: a new red flag in status epilepticus. J Neurol (2024). https://doi.org/10.1007/s00415-024-12603-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12603-x