Abstract
Parkinson’s disease displays clinical heterogeneity, presenting with motor and non-motor symptoms. Heterogeneous phenotypes, named brain-first and body-first, may reflect distinct α-synuclein pathology starting either in the central nervous system or in the periphery. The immune system plays a prominent role in the central and peripheral pathology, with misfolded α-synuclein being placed at the intersection between neurodegeneration and inflammation. Here, we characterized the inflammatory profile and immune-phenotype of peripheral blood mononuclear cells (PBMCs) from Parkinson’s disease patients upon stimulation with α-synuclein monomer or oligomer, and investigated relationships of immune parameters with clinical scores of motor and non-motor symptoms. Freshly isolated PBMCs from 21 Parkinson’s disease patients and 18 healthy subjects were exposed in vitro to α-synuclein species. Cytokine/chemokine release was measured in the culture supernatant by Multiplex Elisa. The immune-phenotype was studied by FACS-flow cytometry. Correlation analysis was computed between immune parameters and parkinsonian motor and non-motor scales. We found that Parkinson’s disease patients exhibited a dysregulated PBMC-cytokine profile, which remained unaltered after exposure to α-synuclein species and correlated with both motor and non-motor severity, with a strong correlation observed with olfactory impairment. Exposure of PBMCs from healthy controls to α-synuclein monomer/oligomer increased the cytokine/chemokine release up to patient’s values. Moreover, the PBMCs immune phenotype differed between patients and controls and revealed a prominent association of the Mos profile with olfactory impairment, and of NK profile with constipation. Results suggest that a deranged PBMC-immune profile may reflect distinct clinical subtypes and would fit with the recent classification of Parkinson’s disease into peripheral-first versus brain-first phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a complex neurodegenerative disorder characterized by a set of cardinal motor features variably associated with several non-motor symptoms (NMS) such as REM sleep behavior disorder, cognitive and mood changes, hyposmia, constipation, cardiovascular disturbances and others [1]. Clinical heterogeneity may reflect PD subtypes with different pathophysiology and pathological progression [2]. The neuropathological hallmark of PD is represented by deposit of Alpha-synuclein (αSyn), a monomeric protein that may also aggregate into toxic species and may be detectable in extracellular biofluids of patients with PD (PWP), including the cerebrospinal fluid (CSF), blood and saliva [3,4,5,6]. The initial αSyn pathology may occur in the brain or in the periphery, an observation that has led to the hypothesis that PD comprises two overall subtypes: a body-first subtype, in which αSyn pathology originates in the enteric nervous system and invades the CNS via the vagus nerve and sympathetic connectome; a brain-first subtype, in which pathology arises in the brain itself, most often in the limbic system or in the olfactory bulb. In this context, constipation has been considered as a prodromal NMS of PD with peripheral-onset, while hyposmia is a prodromal NMS linked to CNS involvement [7, 8].
Several earlier studies showing microgliosis [9, 10] and altered levels of brain’s cytokines [11, 12] have suggested that the immune system can play a pivotal role in PD pathology [13]. More recently, brain infiltrates of peripheral immune cells [14], peripheral inflammation and altered peripheral immune profile have been reported, indicating that immune response is systemically dysregulated [13]. Correlations between blood mononuclear cell (PBMCs) subpopulations or peripheral cytokines production and severity of motor/non-motor symptoms [15,16,17,18,19,20,21,22,23,24,25,26,27,28] also support the relevant role of central and peripheral immune changes in PD.
In the PD brain, αSyn is placed at the intersection between neurodegeneration and inflammatory responses. While neurotoxicity is mostly caused by αSyn aggregates [29,30,31], inflammatory response in microglia can be elicited by both monomeric and aggregated species [32, 33]. Moreover, αSyn may affect peripheral immune cells by stimulating cytokine overproduction, thereby contributing to immune activation [33,34,35,36,37]. These findings notwithstanding, several effects of αSyn monomers and oligomers on peripheral immune response and the relationship of immune changes with motor and non-motor clinical phenomenology remain to be definitely clarified. To investigate the role of monomeric and aggregated forms of α-syn in PD-associated inflammation, we analyzed a large panel of cytokines/chemokines as well as the immune cell profile in PBMCs isolated from PWP and healthy subjects (HS) upon stimulation with an amount of exogenous human αSyn monomer (αSynM) and oligomer (αSynO) described in the plasma of PD patients [38]. Correlations were drawn between PBMCs immune response and measures of motor and non-motor PD severity.
Materials and methods
Participants
PWP were enrolled at the outpatient Movement Disorder Clinic of the University of Cagliari. Diagnosis was made by a movement disorder expert according to the diagnostic criteria from the Movement Disorder Society [39]. Controls were HS attending the same center as caregivers or relatives of non-parkinsonian patients, with no history of PD or other neurodegenerative disorders. HS were clinically evaluated by the same physicians, and they were included in the study if both the neurological exam and the cognitive abilities were normal. None of the HS reported any of the typical prodromal NMSs of PD, such as REM sleep behavior disorder, olfactory deficit, constipation, and mood disorders. Exclusion criteria were atypical parkinsonism, dementia, immunological diseases requiring continuous immunomodulatory therapy, uncontrolled diabetes, recent vaccination against COVID-19 and infections occurring less than 4 weeks prior the recruitment. Motor severity was assessed by the modified Hoehn and Yahr (HY) scale [40] and Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) Scale [41]. The burden of non-motor symptoms was assessed using the Non-Motor Symptoms Scale (NMSS) [42] that allows the identification of specific non-motor symptoms [43]. Total NMSS score and single items score were computed. Cognitive abilities were assessed by the Montreal Cognitive Assessment (MoCA) [44]. Data on current medications and disease duration were also collected. The levodopa equivalent daily dose (LEDD) was computed as previously reported [45]. The study was approved by the Local Ethical Committee (approval n. PG/2021/5461) and performed according to the Declaration of Helsinki. Participants were provided with an explanatory overview of the study and signed their consent to participate.
Exogenous human α-synuclein species synthesis and purification
αSynM. αSynM was obtained through recombinant expression in E. coli using a pT7-7 plasmid, as previously described [29]. Protein was further purified by size exclusion chromatography (Hiload 26/60 Superdex 75 preparation grade, GE Healthcare, Little Chalfont, UK). Protein purity was assessed via SDS-PAGE, and protein concentrations determined spectrophotometrically.
αSynO. αSynO samples were prepared as previously described [29], starting from 6 mg of recombinant acetylated αSynM. αSynO samples were checked with circular dichroism and dynamic light scattering to conform with standard properties identified in our previous structural study [29]. Some samples were also tested for their cytotoxicity in neuronal cells using the MTT test [46]. After the purification procedure αSyn was tested for endotoxin contamination via the LAL (Limulus Amebocyte Lysate) assay (Kairosafe, Italy). The detection for bacterial endotoxin was constantly < 0.06 E.U./ml.
Fluorescent αSyn molecules were labelled with the AF647 dye (Invitrogen, Carlsbad, CA, USA) through ligation with the thiol moiety of Cys 122. Fluorescent oligomers were generated by mixing 90% unlabelled αSyn and 10% AF647-αSyn. The low ratio of labeled/unlabeled monomers and the position of the fluorescent probe in the C-terminal region, outside the structured oligomer core [29], ensured that no significant modifications to the oligomer properties were induced by the labelling protocol, as established by biophysical measurements.
Samples collection and PBMCs isolation from whole blood
Figure 1A summarizes the experimental protocol. Fresh blood samples were collected and diluted (1:1) in Hanks’ Balanced Salt Solution (HBSS). PBMCs were isolated by density gradient media (Ficoll-Paque). The diluted blood was layered on top of an equal volume of Ficoll-Paque and centrifuged at 500 g and 20 °C for 30 min. The PBMC layer was collected and PBS-washed. Cells were counted by automatic Scepter™2.0 counter (Merck Millipore) and 8 × 105/mL were cultured in RPMI supplemented with FBS 10–1% penicillin/streptomycin in 12-well plates, and treated for 24 h with 0.5 µM of αSynO or αSynM [29], or for 2 h with 0.5 µM of αSynO conjugated to FITC. The αSyn concentration was selected based on previous work reporting the αSynO content in peripheral blood of PWP [38].
Cytokine and chemokine analysis by multiplex ELISA
Cytokines and chemokines release was assayed in the supernatant of PBMCs collected 24 h after αSynO/αSynM treatment. Cytokine & Chemokine Convenience 34-Plex Human ProcartaPlex™ Panel (EPXR340-12167–901, Thermo Fisher Scientific) was performed according to the manufacturer’s instructions. Analyte concentration was measured by Luminex MAGPIX (Luminex Corporation, Austin, TX) and data analyzed with xPONENT® software (Luminex Corporation, Austin, TX).
Internalization of human α‐synuclein oligomers
PBMCs treated with FITC-conjugated αSynO were centrifuged at 500 g for 5 min, washed and resuspended in PBS. Cells were incubated with CD45-ECD (1:10, A07784, Beckman Coulter, Brea, CA) for 15 min at RT in the dark to visualize the membrane. The cell suspension was transferred on a slide and images of alive PBMCs were acquired (Olympus BX4, 40 × magnification).
Immunophenotyping of PBMCs by FACS-flow cytometry
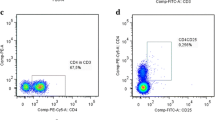
After incubation with αSynO/αSynM cells were collected, centrifuged at 500 g for 5 min, washed and resuspended in PBS. To exclude dead cells PBMCs were stained with the viability dye ViaKrome 405 (1:20, C36614, Beckman Coulter, Brea, CA). A classical FACS gating strategy was used to separate T cells, B cells, Mos and NK cells. To detect surface antigens cells were stained with a panel of specific monoclonal antibodies for 15 min at RT in the dark. The antibodies and relative concentrations were: CD45-KO (1:20, B36294), CD3-APC (1:20, A94680), CD4-PC5 (1:10, B16491), CD8-AF700 (1:10, B76279), CD19-APC (1:20, IM2470), CD14-PC7 (1:20, A22331), CD16-ECD (1:10, B49216), and CD56-PE (1:10, A07788), purchased from Beckman Coulter. After staining, cells were fixed with 1% PFA and analyzed with MoFlo Astrios EQs cell sorter (Beckman Coulter Inc, Brea, CA) with Summit version 6.3.1 software and 405, 488 and 642 nm lasers. Instrument compensation was set using the antibody capture beads kit VersaCom (B22804, Beckman Coulter, Brea, CA) following manufacturer’s instructions.
Figure 1B illustrates the gating strategy. Briefly, starting from CD45 + PBMCs, CD3 + T cells were separated into immune subpopulations based on single or double surface expression of CD4 and CD8 markers. CD3- cells expressing the surface marker CD19 were identified as B cells, while CD19- cells included Mos, NK cells and dendritic cells. Mos were further subdivided based on their expression of CD14 and CD16, into classical (cMos, CD14high/CD16−), intermediate (iMos, CD14high/CD16high), and non-classical (ncMos, CD14low/CD16high) [25, 47]. NK cells (CD14-) were separated based on the CD56 and CD16 expression, into immature NKs (imNK, CD56bright/CD16 +), mature NKs (mNK, CD56dim/CD16 +) and unconventional NKs (ucNK, CD56-/CD16 +) [48, 49].
Statistical analysis
Statistical analysis was performed using Prism 8 (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics for Macintosh, Version 29.0.2.0 (IBM Corp. in Armonk, NY). Data were expressed as means ± standard errors of the means (SEM), and analyzed by parametric or non-parametric tests (unpaired t test with Welch's correction and Mann–Whitney test) and one-way ANOVA followed by Tukey’s Multiple Comparison Test. In immunophenotype experiments, cell frequency data were presented as the percentage respect to each selected PBMCs subpopulation and to the total PBMCs population for each sample. The Spearman’s rank correlation coefficient with two-tailed p values was used to check for correlations between cell frequency in immunophenotype experiments and clinical scores, or between cytokines/chemokines concentration in multiplex ELISA experiments and clinical scores. To check the effect of multiple testing on single correlations, we used the original FDR (false discovery rate) method of Benjamini and Hochberg. A Quade nonparametric ANCOVA test considering age as a covariate, was used to verify whether age or disease duration may affect the differences observed between PD and HS internalization results.
Results
Twenty-one PWP and 18 HS individuals participated into the study, (Table 1). The two groups were similar for sex (10 women and 11 men vs. 8 women and 10 men, p = 1) and age (70.5 ± 8.9 vs. 72.7 ± 7 years, p = 0.4).
Cytokine profile in culture medium from PBMCs
Several inflammatory cytokines (i.e., IL-2, IL-6 and IL-17a), anti-inflammatory cytokines (i.e., IL-4, IL-10 and IL-13) (Fig. 2A) and chemokines (CCL3, CCL4 and CCL2) (Fig. 2B) were significantly higher expressed in the culture media of unstimulated PBMCs from PWP than HS. In vitro exposure to αSynM and αSynO did not modify the cytokine/chemokine release in PBMCs from PWP; by contrast, both αSyn species induced a potent inflammatory response in PBMCs from HS, with the release of cytokines and chemokines increasing to levels similar to those observed in PWP (Fig. 2A-B).
Internalization of fluorescent FITC-conjugated αSynO was significantly lower in PBMCs from PWP than HS (10% vs. 26% of cells, p < 0.001 by Quade nonparametric ANCOVA test considering age as a covariate) (Fig. 3).
Correlation of cytokine/chemokine concentrations and clinical measures
When we checked for possible relationships between cytokine/chemokine concentration from the entire panel and clinical measures, several significant correlations emerged (Table 2) The remaining correlations that failed to reach significance were not shown). Namely, IL-2, IL-5, IL-6 and IL-9 positively correlated with the Q28 parameter of the NMSS; IL-7 positively correlated with UPDRS-III score, total NMSS score, and the Q28 item; IL-18 positively correlated with UPDRS-III score, HY staging and the Q28 item; and the chemokine CXCL8 correlated positively with total NMSS score and the Q28 item.
PBMC immunophenotype
PBMCs immunophenotyping by FACS-flow cytometry yielded similar percentage of viable cells in PWP and HS (98.4 ± 1.10 vs. 97.2 ± 2.7, percent of total isolated PBMCs). Although the small number of subjects involved, the HS and PWP groups were matched for sex, and the sex effect on immune profile was evaluated. Since we did not find any significant sex effect on the immune profile, sexes were merged in graphs.
Monocytes
The percentage of Mos out of CD45 + PBMCs was similar in PWP and HS (supplementary Table 1). Three Mos subpopulations—cMos, iMos and ncMos—were identified according to CD14 and CD16 expressions (Fig. 4A). The frequency of the three subpopulations was comparable in PWP and HS (Fig. 4A). The Mos profile was not affected by treatment with αSynM or αSynO neither in PWP nor in HS (supplementary Table 1).
NKs
NK subpopulations were identified based on CD16 and CD56 expression (Fig. 4B and supplementary Fig. 1). The NK cell percentage out of CD45 + PBMCs was similar in PWP and HS (supplementary Table 1). As expected, the imNKs (CD56 Bright/CD16 +) represented the less frequent subpopulation and were similarly represented in PWP and HS. mNKs (CD56Dim/CD16 +) were the most frequent subpopulation and displayed a tendency to decrease in PWP (p = 0.067) (Fig. 4B). Finally, we observed a third NK subpopulation (CD56-/CD16 +), classified as unconventional NKs (ucNKs) based on previous description (Fig. 4B and supplementary Fig. 1) [48]. ucNKs were highly frequent in PWP but nearly absent in HS (p < 0.05) (Fig. 4B). Stimulation with αSynM or αSynO did not change the NK subpopulations frequency (supplementary Table 1).
T cells and B cells
The percentage of T cells out of CD45 + PBMCs was similar in PWP and HS (supplementary Table 1). Out of the total CD3 + T cell population, CD8 + cells tend to decrease, while CD4 + and double-positive (CD4 + CD8 +) cells tend to increase in PWP (supplementary Fig. 2) as previously reported [22]. Frequency of B cells showed a trending decrease in PWP (supplementary Fig. 2). Stimulation with αSynM or αSynO did not change the frequency of T or B cells (supplementary Table 1).
Correlation of PBMC immunophenotype and clinical measures
When we checked for possible relationships between immune cell subpopulations and motor and non-motor symptoms, several significant correlations emerged.
An inverse correlation was found between cMos frequency and disease duration (Rho = − 0.446; p = 0.049) (Fig. 5A and supplementary Table 2), and between cMos frequency and the NMSS item “olfactory deficits” (Q28 in NMSS) (Rho = − 0.64; p = 0.003) (Fig. 5B and supplementary Table 2). The remaining correlations not reaching statistical significance were not shown. When patients were stratified by the NMSS Q28 item (presence of olfactory symptoms), the frequency of cMos was lower in patients reporting olfactory deficits (p < 0.01) (Fig. 5C). No other correlation between cMos frequency and other NMSS items could be detected.
Spearman correlation between (A) cMos and disease duration and (B) cMos and Q28 item limited to olfactory deficit. C cMos frequency in PWP stratified for olfactory deficit. T test with Welch’s correction, *p < 0.05; **p < 0.01. Spearman correlation between (D) mNKs (CD16 + /CD56dim) or (E) ucNKs (CD16 + /CD56-) and the Q21 item for constipation. F mNKs and ucNKs frequency in PWP stratified for constipation. G mNK/ucNK ratio in PWP stratified for constipation. T test with Welch’s correction, *p < 0.05. PWP: patients with Parkinson’s disease; PDOD Parkinson’s disease patients with olfactory deficits, PDC Parkinson’s disease patients with constipation
The NK frequency did not correlate with any motor item nor with disease duration. However, there was an inverse correlation between mNK frequency and the NMSS Q21 item (constipation) (Rho = − 0.52, p value = 0.021) (Fig. 5D and supplementary Table 2) and a positive correlation between ucNKs and the NMSS Q21 item (Rho = 0.55, p value = 0.014) (Fig. 5E and supplementary Table 2. Remaining correlations that failed to reach significance were not shown). When patients were stratified by the NMSS Q21 item, the frequency of mNKs was lower, and the frequency of ucNKs was higher, in patients reporting constipation (p < 0.01) (Fig. 5F). Accordingly, the mNK/ucNK ratio decreased in patients reporting constipation (Fig. 5G) (p < 0.05). Finally, inflammatory cytokines IL-6, IL-9, IL-13, IL-21, as well the chemokine CCL4 positively correlated with ucNK frequency (p < 0.05; see supplementary Table 3 for details on Rho/p values).
Discussion
This study analyzed the inflammatory profile and phenotype of PBMCs isolated from PWP and healthy individuals, before and after stimulation with αSynM and αSynO. PBMCs from PWP displayed a marked cytokine/chemokine inflammatory profile in the absence of exogenous αSyn stimulation, which correlated with the UPDRS-III score and NMSS total score. Stratifying by single NMSS items yielded a significant correlation with constipation. Stimulation with αSynM and αSynO could not further modify the inflammatory profile of PBMCs from PWP but raised the inflammatory response of PBMC from HS to levels comparable to those observed in unstimulated PBMCs from PWP. We also observed a reduced capacity of PBMCs from PWP to phagocytose αSyn in vitro. The PBMCs immune phenotype differed between PWP and HS. Mos correlated significantly with olfactory impairment, NKs correlated with constipation. Accordingly, stratification of patients by olfactory impairment or constipation revealed significant differences in the frequency of Mos and NK subpopulations, respectively.
The release of higher amounts of pro-inflammatory and anti-inflammatory cytokines and chemokines by unstimulated PBMCs from PWP extends previous reports on PBMCs or serum reporting variable results [11, 12, 17, 50,51,52,53]. The present findings supported a dysregulated peripheral immune response in PD and the possible recruitment of monocytes from periphery to the brain. Among the 30 cytokines and chemokines analyzed, some of them correlated with disease severity as assessed by UPDRS-III score (IL-7, IL-18 and CCL11) and with NMSS total score (IL-7 and CXCL8). Of note, cytokines IL-6, IL-2, IL-5, IL-7, IL-9, IL-18 and the chemokine CXCL8/IL-8 significantly correlated with the NMSS Q28 item (indicating olfactory deficit) but not with other NMSS items. This is a novel information that adds to a few previous studies exploring the association of peripheral cytokines and chemokines with motor and cognitive symptoms [15,16,17, 51, 52, 54,55,56,57]. The herein highlighted relationship between cytokine profile and olfactory impairment, an early sign that typically precedes cardinal PD motor signs, supports a contribution of peripheral inflammation to the pathophysiology of PD [13] and raises the possibility, to be explored, that measuring peripheral inflammatory species would contribute to diagnose prodromal PD.
The baseline cytokine/chemokine profile in PWP could not be modified by a stimulation with oligomeric αSyn at a concentration comparable to that described in the plasma of PD patients [38]. Instead, αSyn stimulation of PBMCs from HS raised the cytokine response to the same qualitative/quantitative level observed in unstimulated PBMCs from PWP. Although the present study was not designed to investigate dose-dependent responses, results may suggest that PBMCs collected from PWP were already highly activated. Moreover, results showed that both species of αSyn were inflammatory triggers for immune cells. This observation fits into the ongoing debate on differential toxicity of αSyn strains. While a number of studies have demonstrated that direct αSyn toxicity against neurons is structure-dependent [29, 58, 59], results were mixed about the inflammatory potential of monomeric and aggregated αSyn species against microglia and PBMCs [31, 37, 60]. This inconsistency could be related to the various degrees of toxicity displayed by diversely aggregated αSyn species used in these studies. Our results are in line with the previous reports showing that monomer and aggregated αSyn elicit a microglial response [37], and that both species bind to TLR2 and activate the downstream pathway [33]. Despite the quite different conformational properties of monomeric and oligomeric αSyn, the TLR2 has the capability to recognize a wide range of structurally unrelated PAMPs and DAMPs. The C-terminal and the N-terminal domains are exposed in oligomeric αSyn, and readily accessible in the unstructured monomeric state, thus potentially providing common interaction sites for TLR2. Therefore, unlike neurons peripheral leukocytes were similarly activated by oligomeric and monomeric αSyn species. Our observation was strengthened by the use of well-characterized human αSyn oligomers which are kinetically trapped in a toxic conformation and highly homogeneous in size and structural properties [29]. The purity of these oligomers previously enabled the characterization of their toxicity mechanisms in vitro and in vivo against neurons and glial cells [61, 62].
The reduced capability of PBMCs from PWP to phagocytose αSyn in vitro was consistent with studies showing a decreased expression of TLR4 in peripheral phagocytes from PWP [25], and studies indicating a reduced capacity of αSyn clearance by glial cells in PD models [61, 63,64,65].
Although the percentage of Mos subpopulations did not differ in PBMCs from PWP and HS, cMos inversely correlated with disease duration and the NMSS item Q28 “olfactory deficits”. This is a further novel information that strengthened the aforementioned association between several cytokines/chemokines and olfactory deficit. Previous studies have shown that Mos are highly dynamic and stage-dependent in PD, showing an increase of cMos in the early disease stage but not at later stages, in line with our results [24, 25]. Moreover, a negative correlation was reported between frequency of cMos and measures of cognitive impairment in PWP [25] supporting a critical involvement of Mos dysregulation in the brain pathology. Our finding of an overproduction of monocyte-chemoattractant chemokines by PBMC from PWP, supports the possibility that chemokines would drive Mos to migrate from blood to the inflamed brain tissue. Other studies reported varying results on Mos profile, likely reflecting the disease stage analyzed and the gating strategy applied [52, 66].
The few studies that have examined changes in peripheral NK frequency in PWP reported uneven results [25, 26, 28, 67,68,69,70], probably due to pronounced differences in the markers used to identify these cells. Our characterization of NK profile based on classical CD56/CD16 expression [71] yielded the identification of three subpopulations, namely immature NKs, terminally mature and cytotoxic NKs, and a third subpopulation categorized as unconventional NKs. Typically, unconventional NKs increase during viral infections or autoimmune diseases [48, 49, 72], with their expansion being associated with a decrease in the mature subpopulation and regulated by cytokines [48, 49]. Consistently, we found a positive correlation between cytokines production in PWP and the frequency of ucNKs. Notably, the unconventional NK subset was significantly more represented among PWP while the mature subset was significantly more present in HS. Moreover, this is the first report describing an inverse specific correlation of both mature and unconventional NKs with constipation. Hence, mNK and ucNKs were respectively low and highly represented in a subgroup of PWP reporting constipation, and inversely correlated with this symptom. This report adds to the few studies that investigated NK cell frequency in relation with other PD clinical features [73,74,75]. Interestingly, multiple lines of evidence suggest a relationship between viral or bacterial exposures, alterations in gut microbiota, and the increased risk of developing PD [13]. Our finding further supports the specific involvement of the NK population in PD phenotypes with gut disturbances.
The clinical heterogeneity of PD may reflect subtypes with distinct pathophysiology and progression, namely peripheral-first versus brain-first phenotype, whose differential diagnosis would benefit of specific biological parameters. While the body-first subtype implies that pathology originates in periphery, including the enteric nervous system, and subsequently invades the CNS, the brain-first subtype implies that pathology arises in the brain itself, most often in the limbic system or in the olfactory bulb. In this context, constipation has been proposed as a prodromal symptom reflecting peripheral-onset, while prodromal hyposmia may reflect CNS onset [7, 8]. The present finding of correlations between specific immune cell populations and specific NMS of PD well fits into this scenario and may aid the early differential diagnosis of peripheral versus brain-onset phenotypes, although larger population-based studies are warranted to consolidate our findings. Hence, the NK profile was mostly affected by constipation and may support the early recognition of body-first PD phenotypes, while the Mos profile changed in relation to olfactory deficits and severity/duration of motor symptoms, supporting recognition of brain-first phenotypes.
This study has strengths and limitations. The research was conducted on a limited number of PWP, and a selection bias cannot be ruled out. Nevertheless, the inclusion of consecutive patients throughout the study period and their diagnosis by movement disorder specialists of the same center, following the same study protocol, provided a sample reflective of the typical PD population. We believe that PD patients in our study were relatively homogeneous in terms of disease stage. Indeed, this is supported by the low variability in the HY stage and the UPDRS scores, which are values of a consistent disease severity across participants. Our control group was composed solely of HS, whereas others also considered patients with a variety of neurological conditions mimicking PD, such as dementia with Lewy Bodies and multiple system atrophy. To assess NMDS, we used the NMSS, a widely used tool that is based on patient self-reporting and may thus be influenced by the individual's perception and understanding of their symptoms. Expanding our results with specific questionnaires, such as the semi-objective olfactory evaluation with the Sniffin' Sticks test or the University of Pennsylvania Smell Identification Test would be needed.
PBMCs were freshly analyzed to avoid any freeze and thaw cycle which may affect phenotyping and the relative subpopulation percentage. To avoid any methodological bias and allow data comparison, all samples were immediately processed after collection and equally isolated by density gradient media. Although it has been reported that the expression of PBMC cell surface markers differ across cell isolation procedures and upon cell culture respect to direct ex-vivo measurement [76, 77], cell culture was mandatory to test the effect of αSyn monomer and oligomer. A similar protocol was used in the previous studies involving PBMC cultures [37].
Importantly, we took advantage of structurally characterized and highly homogeneous human αSyn oligomers to stimulate PBMCs. In addition, the multiplex Elisa test used in the present study enabled the assessment of a wide range of cytokines and chemokines in a single well and in the same sample aliquot, thus overcoming caveats arising from different individual cytokine assays comparison. Owing to the small size of the sample and the low statistical power, correlation analysis for most NMSS items yielded inconclusive results. Nevertheless, the significance level reached by some correlation analyses including the NMSS Q28 and Q21 items, despite of low study power, would suggest a greater magnitude of the association with some immune parameters for the Q28 or Q21 NMSS items than for the other items.
Conclusion
Our findings provided new insights on immune response in PWP. While confirming and extending several deviations in peripheral immune profile of PWP, the significant correlations between some immune and clinical parameters unveil an uneven behavior of immune subpopulations in relation with specific NMS. The prevalent association of the Mos profile with olfactory impairment and the association of NK profile with a peripheral NMS such as constipation would fit with the recent classification of PD into subtypes with different pathological onset, namely peripheral versus brain-first phenotype. In the context of the clinical heterogeneity of PD, measurement of peripheral immune parameters may aid to differentiate peripheral versus brain-onset phenotypes. Our findings also highlight the potential relevance of peripheral inflammatory parameters in delineating prodromal PD.
References
Chaudhuri KR, Naidu Y (2008) Early Parkinson’s disease and non-motor issues. J Neurol 255(Suppl 5):33–38. https://doi.org/10.1007/s00415-008-5006-1
Berg D, Borghammer P, Fereshtehnejad S-M et al (2021) Prodromal Parkinson disease subtypes—key to understanding heterogeneity. Nat Rev Neurol 17:349–361. https://doi.org/10.1038/s41582-021-00486-9
Angius F, Mocci I, Ercoli T et al (2023) Combined measure of salivary alpha-synuclein species as diagnostic biomarker for Parkinson’s disease. J Neurol 270:5613–5621. https://doi.org/10.1007/s00415-023-11893-x
Mollenhauer B, Cullen V, Kahn I et al (2008) Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol 213:315–325. https://doi.org/10.1016/j.expneurol.2008.06.004
Vivacqua G, Suppa A, Mancinelli R et al (2019) Salivary alpha-synuclein in the diagnosis of Parkinson’s disease and progressive supranuclear palsy. Parkinsonism Relat Disord 63:143–148. https://doi.org/10.1016/j.parkreldis.2019.02.014
Ganguly U, Singh S, Pal S et al (2021) Alpha-synuclein as a biomarker of parkinson’s disease: good, but not good enough. Front Aging Neurosci 13:702639. https://doi.org/10.3389/fnagi.2021.702639
Heinzel S, Aho VTE, Suenkel U et al (2021) Gut microbiome signatures of risk and prodromal markers of parkinson disease. Ann Neurol 90:E1–E12. https://doi.org/10.1002/ana.26128
Metta V, Leta V, Mrudula KR et al (2022) Gastrointestinal dysfunction in Parkinson’s disease: molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J Neurol 269:1154–1163. https://doi.org/10.1007/s00415-021-10567-w
Imamura K, Hishikawa N, Sawada M et al (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106:518–526. https://doi.org/10.1007/s00401-003-0766-2
Gerhard A (2016) TSPO imaging in parkinsonian disorders. Clin Transl Imaging 4:183–190. https://doi.org/10.1007/s40336-016-0171-1
Mogi M, Harada M, Narabayashi H et al (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 211:13–16. https://doi.org/10.1016/0304-3940(96)12706-3
Mogi M, Kondo T, Mizuno Y, Nagatsu T (2007) p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett 414:94–97. https://doi.org/10.1016/j.neulet.2006.12.003
Tansey MG, Wallings RL, Houser MC et al (2022) Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol 22:657–673. https://doi.org/10.1038/s41577-022-00684-6
Brochard V, Combadière B, Prigent A et al (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119:182–192. https://doi.org/10.1172/JCI36470
Fu J, Chen S, Liu J et al (2023) Serum inflammatory cytokines levels and the correlation analyses in Parkinson’s disease. Front Cell Dev Biol 11:1104393. https://doi.org/10.3389/fcell.2023.1104393
Karpenko MN, Vasilishina AA, Gromova EA et al (2018) Interleukin-1β, interleukin-1 receptor antagonist, interleukin-6, interleukin-10, and tumor necrosis factor-α levels in CSF and serum in relation to the clinical diversity of Parkinson’s disease. Cell Immunol 327:77–82. https://doi.org/10.1016/j.cellimm.2018.02.011
Reale M, Iarlori C, Thomas A et al (2009) Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun 23:55–63. https://doi.org/10.1016/j.bbi.2008.07.003
Qin X-Y, Zhang S-P, Cao C et al (2016) Aberrations in peripheral inflammatory cytokine levels in parkinson disease: a systematic review and meta-analysis. JAMA Neurol 73:1316–1324. https://doi.org/10.1001/jamaneurol.2016.2742
Bhatia D, Grozdanov V, Ruf WP et al (2021) T-cell dysregulation is associated with disease severity in Parkinson’s Disease. J Neuroinflammation 18:250. https://doi.org/10.1186/s12974-021-02296-8
Galiano-Landeira J, Torra A, Vila M, Bové J (2020) CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 143:3717–3733. https://doi.org/10.1093/brain/awaa269
He Y, Peng K, Li R et al (2022) Changes of T lymphocyte subpopulations and their roles in predicting the risk of Parkinson’s disease. J Neurol 269:5368–5381. https://doi.org/10.1007/s00415-022-11190-z
Kouli A, Camacho M, Allinson K, Williams-Gray CH (2020) Neuroinflammation and protein pathology in Parkinson’s disease dementia. Acta Neuropathol Commun 8:211. https://doi.org/10.1186/s40478-020-01083-5
Storelli E, Cassina N, Rasini E et al (2019) Do Th17 lymphocytes and IL-17 contribute to parkinson’s disease? a systematic review of available evidence. Front Neurol 10:13. https://doi.org/10.3389/fneur.2019.00013
Wijeyekoon RS, Kronenberg-Versteeg D, Scott KM et al (2020) Peripheral innate immune and bacterial signals relate to clinical heterogeneity in Parkinson’s disease. Brain Behav Immun 87:473–488. https://doi.org/10.1016/j.bbi.2020.01.018
Konstantin Nissen S, Farmen K, Carstensen M et al (2022) Changes in CD163+, CD11b+, and CCR2+ peripheral monocytes relate to Parkinson’s disease and cognition. Brain Behav Immun 101:182–193. https://doi.org/10.1016/j.bbi.2022.01.005
Cen L, Yang C, Huang S et al (2017) Peripheral lymphocyte subsets as a marker of parkinson’s disease in a chinese population. Neurosci Bull 33:493–500. https://doi.org/10.1007/s12264-017-0163-9
Menees KB, Lee J-K (2022) New insights and implications of natural killer cells in parkinson’s disease. J Parkinsons Dis 12:S83–S92. https://doi.org/10.3233/JPD-223212
Mihara T, Nakashima M, Kuroiwa A et al (2008) Natural killer cells of Parkinson’s disease patients are set up for activation: a possible role for innate immunity in the pathogenesis of this disease. Parkinsonism Relat Disord 14:46–51. https://doi.org/10.1016/j.parkreldis.2007.05.013
Fusco G, Chen SW, Williamson PTF et al (2017) Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 358:1440–1443. https://doi.org/10.1126/science.aan6160
Yasuda T, Nakata Y, Mochizuki H (2013) α-Synuclein and neuronal cell death. Mol Neurobiol 47:466–483. https://doi.org/10.1007/s12035-012-8327-0
Zhang W, Wang T, Pei Z et al (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19:533–542. https://doi.org/10.1096/fj.04-2751com
Dohgu S, Takata F, Matsumoto J et al (2019) Monomeric α-synuclein induces blood-brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc Res 124:61–66. https://doi.org/10.1016/j.mvr.2019.03.005
Codolo G, Plotegher N, Pozzobon T et al (2013) Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 8:e55375. https://doi.org/10.1371/journal.pone.0055375
Earls RH, Menees KB, Chung J et al (2020) NK cells clear α-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of α-synucleinopathy. Proc Natl Acad Sci U S A 117:1762–1771. https://doi.org/10.1073/pnas.1909110117
Lindestam Arlehamn CS, Dhanwani R, Pham J et al (2020) α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat Commun 11:1875. https://doi.org/10.1038/s41467-020-15626-w
Sulzer D, Alcalay RN, Garretti F et al (2017) T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546:656–661. https://doi.org/10.1038/nature22815
Piancone F, Saresella M, La Rosa F et al (2021) Inflammatory responses to monomeric and aggregated α-synuclein in peripheral blood of parkinson disease patients. Front Neurosci 15:639646. https://doi.org/10.3389/fnins.2021.639646
Wang X, Chi J, Huang D et al (2020) α-synuclein promotes progression of Parkinson’s disease by upregulating autophagy signaling pathway to activate NLRP3 inflammasome. Exp Ther Med 19:931–938. https://doi.org/10.3892/etm.2019.8297
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression, and mortality. Neurology 17:427–427. https://doi.org/10.1212/WNL.17.5.427
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease (2003) The unified parkinson’s disease rating scale (UPDRS): status and recommendations. Mov Disord 18:738–750. https://doi.org/10.1002/mds.10473
Chaudhuri KR, Martinez-Martin P, Schapira AHV et al (2006) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord 21:916–923. https://doi.org/10.1002/mds.20844
Goldman JG, Postuma R (2014) Premotor and non-motor features of Parkinson’s disease. Curr Opin Neurol 27:434–441. https://doi.org/10.1097/WCO.0000000000000112
Nasreddine ZS, Phillips NA, Bédirian V et al (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Tomlinson CL, Stowe R, Patel S et al (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653. https://doi.org/10.1002/mds.23429
Cascella R, Perni M, Chen SW et al (2019) Probing the origin of the toxicity of oligomeric aggregates of α-synuclein with antibodies. ACS Chem Biol 14:1352–1362. https://doi.org/10.1021/acschembio.9b00312
Abeles RD, McPhail MJ, Sowter D et al (2012) CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi) /CD16(neg) monocytes: expansion of CD14(hi) /CD16(pos) and contraction of CD14(lo) /CD16(pos) monocytes in acute liver failure. Cytometry A 81:823–834. https://doi.org/10.1002/cyto.a.22104
Mavilio D, Lombardo G, Benjamin J et al (2005) Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A 102:2886–2891. https://doi.org/10.1073/pnas.0409872102
Wang Y, Lifshitz L, Silverstein NJ et al (2023) Transcriptional and chromatin profiling of human blood innate lymphoid cell subsets sheds light on HIV-1 pathogenesis. EMBO J 42:e114153. https://doi.org/10.15252/embj.2023114153
Brodacki B, Staszewski J, Toczyłowska B et al (2008) Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett 441:158–162. https://doi.org/10.1016/j.neulet.2008.06.040
Qu Y, Li J, Qin Q et al (2023) A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Parkinsons Dis 9:18. https://doi.org/10.1038/s41531-023-00449-5
Xiromerisiou G, Marogianni C, Lampropoulos IC et al (2022) Peripheral inflammatory markers TNF-α and CCL2 revisited: association with parkinson’s disease severity. Int J Mol Sci 24:264. https://doi.org/10.3390/ijms24010264
Grozdanov V, Bliederhaeuser C, Ruf WP et al (2014) Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol 128:651–663. https://doi.org/10.1007/s00401-014-1345-4
Williams-Gray CH, Wijeyekoon R, Yarnall AJ et al (2016) Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov Disord 31:995–1003. https://doi.org/10.1002/mds.26563
Rathnayake D, Chang T, Udagama P (2019) Selected serum cytokines and nitric oxide as potential multi-marker biosignature panels for Parkinson disease of varying durations: a case-control study. BMC Neurol 19:56. https://doi.org/10.1186/s12883-019-1286-6
Pessoa Rocha N, Reis HJ, Vanden Berghe P, Cirillo C (2014) Depression and cognitive impairment in Parkinson’s disease: a role for inflammation and immunomodulation? NeuroImmunoModulation 21:88–94. https://doi.org/10.1159/000356531
Yacoubian TA, Fang Y-HD, Gerstenecker A et al (2023) Brain and Systemic Inflammation in De Novo Parkinson’s Disease. Mov Disord 38:743–754. https://doi.org/10.1002/mds.29363
Cascella R, Chen SW, Bigi A et al (2021) The release of toxic oligomers from α-synuclein fibrils induces dysfunction in neuronal cells. Nat Commun 12:1814. https://doi.org/10.1038/s41467-021-21937-3
Emin D, Zhang YP, Lobanova E et al (2022) Small soluble α-synuclein aggregates are the toxic species in Parkinson’s disease. Nat Commun 13:5512. https://doi.org/10.1038/s41467-022-33252-6
Grozdanov V, Bousset L, Hoffmeister M et al (2019) Increased immune activation by pathologic α-synuclein in parkinson’s disease. Ann Neurol 86:593–606. https://doi.org/10.1002/ana.25557
Boi L, Pisanu A, Palmas MF et al (2020) Modeling parkinson’s disease neuropathology and symptoms by intranigral inoculation of preformed human α-synuclein oligomers. Int J Mol Sci 21:8535. https://doi.org/10.3390/ijms21228535
Palmas MF, Etzi M, Pisanu A et al (2022) The intranigral infusion of human-alpha synuclein oligomers induces a cognitive impairment in rats associated with changes in neuronal firing and neuroinflammation in the anterior cingulate cortex. Cells 11:2628. https://doi.org/10.3390/cells11172628
Carta AR, Boi L, Pisanu A et al (2020) Advances in modelling alpha-synuclein-induced Parkinson’s diseases in rodents: virus-based models versus inoculation of exogenous preformed toxic species. J Neurosci Methods 338:108685. https://doi.org/10.1016/j.jneumeth.2020.108685
Fellner L, Stefanova N (2013) The role of glia in α-synucleinopathies. Mol Neurobiol 47:575–586. https://doi.org/10.1007/s12035-012-8340-3
Stefanova N, Fellner L, Reindl M et al (2011) Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol 179:954–963. https://doi.org/10.1016/j.ajpath.2011.04.013
Schlachetzki JCM, Prots I, Tao J et al (2020) Author Correction: a monocyte gene expression signature in the early clinical course of Parkinson’s disease. Sci Rep 10:6261. https://doi.org/10.1038/s41598-020-62928-6
Niwa F, Kuriyama N, Nakagawa M, Imanishi J (2012) Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr Gerontol Int 12:102–107. https://doi.org/10.1111/j.1447-0594.2011.00740.x
Stevens CH, Rowe D, Morel-Kopp M-C et al (2012) Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol 252:95–99. https://doi.org/10.1016/j.jneuroim.2012.07.015
Sun C, Zhao Z, Yu W et al (2019) Abnormal subpopulations of peripheral blood lymphocytes are involved in Parkinson’s disease. Ann Transl Med 7:637. https://doi.org/10.21037/atm.2019.10.105
Huang Y, Liu H, Hu J et al (2021) Significant difference of immune cell fractions and their correlations with differential expression genes in parkinson’s disease. Front Aging Neurosci 13:686066. https://doi.org/10.3389/fnagi.2021.686066
Khalil M, Malarkannan S (2022) Innatus immunis: evolving paradigm of adaptive NK cells. J Exp Med 219:e20221254. https://doi.org/10.1084/jem.20221254
Cao W-J, Zhang X-C, Wan L-Y et al (2021) Immune dysfunctions of CD56neg NK cells are associated With HIV-1 disease progression. Front Immunol 12:811091. https://doi.org/10.3389/fimmu.2021.811091
Farmen K, Nissen SK, Stokholm MG et al (2021) Monocyte markers correlate with immune and neuronal brain changes in REM sleep behavior disorder. Proc Natl Acad Sci U S A 118:e2020858118. https://doi.org/10.1073/pnas.2020858118
Tian J, Dai S-B, Jiang S-S et al (2022) Specific immune status in Parkinson’s disease at different ages of onset. NPJ Parkinsons Dis 8:5. https://doi.org/10.1038/s41531-021-00271-x
Anderson KM, Augusto DG, Dandekar R et al (2020) Killer cell immunoglobulin-like receptor variants are associated with protection from symptoms associated with more severe course in parkinson disease. J Immunol 205:1323–1330. https://doi.org/10.4049/jimmunol.2000144
Rokita E, Menzel EJ (1997) Characteristics of CD14 shedding from human monocytes. Evidence for the competition of soluble CD14 (sCD14) with CD14 receptors for lipopolysaccharide (LPS) binding. APMIS 105:510–518. https://doi.org/10.1111/j.1699-0463.1997.tb05048.x
Kiefer J, Zeller J, Bogner B et al (2021) An unbiased flow cytometry-based approach to assess subset-specific circulating monocyte activation and cytokine profile in whole blood. Front Immunol 12:641224. https://doi.org/10.3389/fimmu.2021.641224
Funding
Open access funding provided by Università degli Studi di Cagliari within the CRUI-CARE Agreement. This work was supported by the Michael J. Fox Foundation for Parkinson’s Research, grant ID 001133; European Research Council (ERC) 819644 BioDisOrder.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vega-Benedetti, A.F., Porcedda, C., Ercoli, T. et al. Immune responses to oligomeric α-synuclein in Parkinson’s disease peripheral blood mononuclear cells. J Neurol (2024). https://doi.org/10.1007/s00415-024-12554-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12554-3