Abstract
Background
Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS) and Spastic Paraplegia Type 7 (SPG7) are paradigmatic spastic ataxias (SPAX) with suggested white matter (WM) involvement. Aim of this work was to thoroughly disentangle the degree of WM involvement in these conditions, evaluating both macrostructure and microstructure via the analysis of diffusion MRI (dMRI) data.
Material and methods
In this multi-center prospective study, ARSACS and SPG7 patients and Healthy Controls (HC) were enrolled, all undergoing a standardized dMRI protocol and a clinimetrics evaluation including the Scale for the Assessment and Rating of Ataxia (SARA). Differences in terms of WM volume or global microstructural WM metrics were probed, as well as the possible occurrence of a spatially defined microstructural WM involvement via voxel-wise analyses, and its correlation with patients’ clinical status.
Results
Data of 37 ARSACS (M/F = 21/16; 33.4 ± 12.4 years), 37 SPG7 (M/F = 24/13; 55.7 ± 10.7 years), and 29 HC (M/F = 13/16; 42.1 ± 17.2 years) were analyzed. While in SPG7, only a mild mean microstructural damage was found compared to HC, ARSACS patients present a severe WM involvement, with a reduced global volume (p < 0.001), an alteration of all microstructural metrics (all with p < 0.001), without a spatially defined pattern of damage but with a prominent involvement of commissural fibers. Finally, in ARSACS, a correlation between microstructural damage and SARA scores was found (p = 0.004).
Conclusion
In ARSACS, but not SPG7 patients, we observed a complex and multi-faced involvement of brain WM, with a clinically meaningful widespread loss of axonal and dendritic integrity, secondary demyelination and, overall, a reduction in cellularity and volume.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spastic ataxia (SPAX) represents a clinical phenotype characterized by the presence of cerebellar ataxia along with spasticity and other pyramidal features [1, 2]. This phenotype is associated with a heterogeneous group of predominantly genetic conditions [3]. Among these, Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS, MIM #270550) and Spastic Paraplegia Type 7 (SPG7, MIM #607259) represent some of the most common forms of autosomal recessive cerebellar ataxias reported in literature, and might, therefore, be used as good models to understand more of the pathophysiological mechanisms behind the development of brain damage in genetically determined SPAX [2, 4].
In this light, magnetic resonance imaging (MRI) plays an unquestionable role in the evaluation of patients with neurodegenerative disorders, including SPAX patients, not only for clinical purposes but also by providing quantitative, reliable, and in vivo information on neuronal damage [5, 6] during the disease course. Among all the sequences available, diffusion MRI (dMRI) is a quantitative technique able to capture subtle but meaningful changes occurring in several conditions, from neurodevelopmental to neurodegenerative, as well as psychiatric or neuroinflammatory disorders [7,8,9,10,11,12]. This method, sensitive to water diffusion characteristics in brain tissue, represent a marker of white matter (WM) microstructural integrity [13], with different parameters that can be extracted from a single sequence but that can provide simultaneously complementary information about neuronal integrity [14, 15]. Some scattered descriptions about a certain degree of WM involvement, using dMRI, have been previously reported in both ARSACS and SPG7 patients [16,17,18,19]. Nevertheless, this information is derived from studies with very small sample size (or even single case reports in SPG7) and relatively outdated processing methods, therefore significantly limiting our comprehension of possible, more profound, pathophysiological changes that might occur in the WM of patients with these disorders.
In this study, we aimed to expand the current knowledge about WM involvement in SPAX patients leveraging the largest standardized dataset of ARSACS and SPG7 patients available to date, collected within the PROSPAX consortium, which is an international multi-center collaborative research project on SPAX [20]. The main aim of this work was to thoroughly disentangle the degree of WM involvement in these two conditions, through a “top to bottom” approach from macrostructure to microstructure. Specifically, we aimed to assess if there were differences between ARSACS, SPG7 patients, and Healthy Controls (HC) in terms of: (1) WM volume, (2) global microstructural WM metrics, and (3) if spatially defined differences of microstructural WM metrics are present. Finally, (4) we correlated microstructural WM changes and detailed clinimetrics to derive more precise understanding of imaging patterns in SPAX.
Materials and methods
Compliance with ethical standards
Data analyzed in this study were acquired within the multi-center project PROSPAX (“An integrated multi-modal progression chart in spastic ataxias”-ClinicalTrials.gov no: NCT04297891). The study was approved by local Ethics Committee of each center and written informed consent was obtained from each participant.
Participants
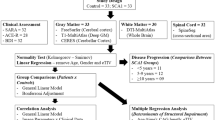
In this multi-center prospective study performed in 8 centers from 6 countries from January 2021 to October 2022, 120 ARSACS patients and 141 SPG7 genetically confirmed patients, along with 77 Healthy Controls (HC) with no history of neurological or psychiatric disorders, were enrolled. All subjects with contraindication or unwillingness to undergo a brain MRI scan were then excluded from this study. After the application of this first exclusion criteria, subjects with an incomplete MRI acquisition (namely, without a T1-weighted volume and a dMRI sequence acquired within the same MR session) or with a poor quality of images (e.g., due to motion artifacts or data corruption) were then further excluded from the study (Fig. 1). For the assessment of disease severity, a neurologic examination within 1 month from the MRI acquisition was performed for each subject via the Scale for the Assessment and Rating of Ataxia (SARA) [21], the Spastic Paraplegia Rating Scale (SPRS) [22] (with the record of the corresponding “functional mobility” items from 1 to 6—fSPRS) and the Friedreich’s Ataxia Rating Scale (FARS) [23]. Furthermore, the Montreal Cognitive Assessment (MoCA) test was also administered to provide a basic cognitive assessment of these patients [24].
MRI data acquisition
All sites acquired a standardized harmonized dMRI protocol on a 3 T scanner with the following parameters: TR = 4200 ms; TE = 90 ms; flip angle = 90°; voxel size = 2 × 2 × 2 mm3 with 3 directions at b = 300 s/mm2, 6 directions at b = 700 s/mm2, 32 directions at b = 1000 s/mm2, 50 directions at b = 2000 s/mm2 in addition to 7 directions at b = 0 s/mm2; GRAPPA acceleration factor = 2; phase encoding = AP; bandwidth = 1780 Hz/pixel). Another acquisition with the same parameters, but with inverse phase encoding direction (PA) and 3 directions at b = 0 s/mm2 values, has been acquired for distortion correction purposes. Along with this standardized dMRI protocol, for all subjects, a 3D-T1-weighted volume was acquired on a sagittal plane with high resolution (voxel size ≤ 1mm3). A complete list of all the scanners used at each site, along with the sequence parameters for the 3D-T1-weighted volume, is available in Supplementary Materials.
MRI data analysis
The 3D-T1-weighted images were segmented in (1) gray matter, (2) sub-cortical gray matter, (3) white matter, (4) cerebrospinal fluid, (5) eventual pathological tissue (absent in all cases) using the 5ttgen command in MRtrix3 and the fsl algorithm [25, 26].
All dMRI images were denoised [27, 28] and pre-processed using the FMRIB Software Library (FSL version 6.0) toolbox (http://www.fmrib.ox.ac.uk/fsl), using the eddy and topup distortion correction commands [29,30,31]. Although a standardized sequence was acquired in all sites, to further remove any possible additional variance caused by site differences, the ComBat data harmonization method was used (https://github.com/Jfortin1/ComBatHarmonization [32]).
Diffusion tensor model was fitted for each subject using b ≤ 1000 s/mm2 data [33], with fractional anisotropy (FA), mean diffusivity (MD), and radial diffusivity (RD) maps that were computed from this model [34]. Along with this approach, the Neurite Orientation Dispersion and Density Imaging (NODDI) model [35] was also fitted (using the Accelerated Microstructure Imaging via Convex Optimization—AMICO—software [36]) to obtain the neurite density index (NDI) maps, that was then weighted by the tissue fraction (NDIw) to avoid any bias in the microstructural map [37].
All processing steps were controlled, and case-by-case reviewed by an experienced user, to ensure the quality of the output.
Statistical analyses
Between group differences—clinico-demographic and global WM evaluation analyses
Possible differences in terms of age, sex, and clinical scores between patients and controls were tested via Wilcoxon rank sum and Pearson’s Chi-squared tests, respectively.
Between-groups differences (SPG7 vs. HC, and ARSACS vs. HC) in terms of global volume and mean WM microstructure were tested via a robust linear regression using age and sex as covariates (along with the total intracranial volume for the global volume analysis, to take into account for head size).
Furthermore, to investigate a possible role of genotype in determining the observed MRI phenotype of WM damage, an ancillary analysis was performed with the same approach comparing WM variables in patients with a different genetic profile.
All these analyses were performed using RStudio software (https://www.R-project.org), with a significance level set for p < 0.05.
Between group differences—voxel-wise analysis
Possible voxel-wise differences of the investigated microstructural measures (namely, FA, MD, RD, and NDIw) between the patients and controls (namely, ARSACS vs. HC and SPG7 vs. HC) were probed using the Tract-Based Spatial Statistics (TBSS) analysis [13]. In particular, each map was co-registered to the FMRIB58_FA template available in FSL, in the Montreal Neurological Institute (MNI) space, using FNIRT’s nonlinear registration tool [38]. Normalized FA maps, visually assessed to ensure good quality of the normalization, were then used to create a WM “skeleton” that represents the alignment-invariant tracts in common to all subjects, applying a thinning algorithm to the average FA map with a threshold set equal to 0.2 [13]. Finally, each subject’s microstructural maps were projected onto this skeleton for subsequent between-group analyses, all age and sex corrected, using the FSL’s randomise tool (number of permutations = 5000). All results were considered significant for p < 0.05. Corrected for multiple comparisons using a Threshold-Free Cluster Enhancement (TFCE) approach [13].
Finally, to evaluate the clinical counterparts of the observed microstructural changes in ARSACS and SPG7 compared to HC, possible correlations between FA values (as the main index of microstructural damage [39, 40], disease duration (calculated as the difference between age at onset and MR date) and clinical scores were also probed via voxel-wise analyses, using the same approach and a statistical threshold described before. In addition, analyses were also probed adding the WM volume as additional covariate, to test whether the possible observed correlations were influenced by WM macrostructural changes.
Results
After application of inclusion and exclusion criteria, a final number of 103 subjects from 5 sites were included in this study, with MRI data of 37 ARSACS patients (M/F = 21/16, mean age = 33.4 ± 12.4 years), 37 SPG7 patients (M/F = 24/13, mean age = 55.7 ± 10.7 years), and 29 HC (M/F = 13/16, mean age = 42.1 ± 17.2 years) that were analyzed (Fig. 1). Demographic and clinical data of the subjects included in this study are available in Table 1, with a breakdown of these variables divided per site available in the Supplementary Table 1.
When evaluating possible differences in terms of global WM metrics, ARSACS patients showed a significant reduction of WM volume compared to HC (719.6 ± 83.7 vs. 809.9 ± 75.9 mL, p < 0.001), while a similar but less pronounced difference that emerged when SPG7 patients and HC were compared (793.9 ± 84.2 vs. 809.9 ± 75.9 mL, p = 0.01) (Fig. 2A). Similarly, a different behavior between ARSACS and SPG7 patients was found for all mean microstructural WM metrics. Indeed, while ARSACS patients showed changes in all the dMRI-derived metrics probed (p < 0.001 for all dMRI metrics), SPG7 showed an increase in mean MD (0.83 ± 0.027 vs. 0.81 ± 0.018 × 10−3 mm2/s, p = 0.006) and RD (0.69 ± 0.029 vs. 0.67 ± 0.022 × 10−3 mm2/s, p = 0.01) values compared to HC, while no differences emerged for FA (p = 0.21) and NDIw (p = 0.09) values. The ancillary analysis investigating the possible role of genetic profile in determining the observed MRI phenotype of WM damage showed no significant differences between subjects harboring one or two truncated alleles and those with a missense mutation of the affected gene (Supplementary Table 2).
Violin plots showing the differences between the three groups in terms of WM volume (A) and global mean microstructural metrics (FA, MD, RD, and NDIw in B–E, respectively). WM = white matter; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; NDIw = neurite density index weighted by tissue fraction
A complete list of the results of the global WM evaluation analyses is available in Table 2, with a graphical representation shown in Fig. 2.
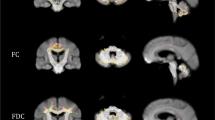
When possible voxel-wise differences in terms of microstructural WM metrics between the three groups were evaluated, we found widespread changes affecting the WM of ARSACS patients, without a spatially distributed pattern of damage but with a severe involvement of commissural fibers (such as the corpus callosum and the fornix) and of the infratentorial structures (such as the pons and the middle cerebellar peduncles) (Fig. 3). In line with the results of the global WM analysis, SPG7 patients showed no significant voxel-wise differences in terms of WM microstructure compared to HC.
Results of the TBSS analysis showing the spatial distribution of microstructural damage in ARSACS patients. Significant differences, in a yellow–red color code scale superimposed to the WM “skeleton” in green, are shown for anatomic reference on selected axial slices of the FA template in the standard MNI space. TBSS = Tract-Based Spatial Statistics; MNI = Montreal Neurological Institute; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; NDIw = neurite density index weighted by tissue fraction
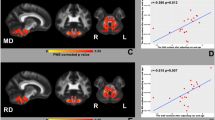
Finally, when possible correlations between clinical scores and WM microstructure were probed, we observed a negative correlation between SARA scores and FA values (p = 0.004) in ARSACS patients, with a more significant involvement of the commissural fibers, and in particular the genu of the corpus callosum (Fig. 4A). These results remained significant after correction for WM volume, suggesting their independence from atrophy (Fig. 4B). No significant correlation emerged between FA values and diseased duration (p = 0.22) or the remaining clinical scales (p = 0.14 and p = 0.64 for SPRS and FARS, respectively), with the exception of two borderline correlations with fSPRS and MoCA scores (p = 0.05 for both tests).
Results of the voxel-wise correlation analysis between microstructural integrity of the WM and clinical scores, showing the spatial distribution of correlation between FA values and SARA scores without (A) and with (B) correction for WM volume. Voxel of significant correlations, in a yellow–red color code scale superimposed to the WM “skeleton” in green, are shown for anatomic reference on the FA template in the standard MNI space in selected coronal (upper row) and axial (lower row) slices. MNI = Montreal Neurological Institute; FA = fractional anisotropy; WM = white matter; SARA = Scale for the Assessment and Rating of Ataxia
Discussion
In this study, we performed a thorough evaluation of WM involvement in one of the largest groups of ARSACS and SPG7 patients using a standardized and harmonized MRI protocol among five different centers worldwide. Our results showed that while subjects with ARSACS present considerable WM involvement both at a macrostructural and microstructural level, SPG7 patients have relatively preserved WM macrostructure and microstructure.
With reference to the findings in ARSACS, we proved a severe macrostructural WM involvement (suggested by a global WM volume loss), coupled to profound and widespread microstructural changes (with changes in all dMRI-derived measures we evaluated). To date, most of the currently available dMRI literature in ARSACS has been focused on the evaluation, using diffusion tensor imaging (DTI), of possible corticospinal tract (CST) and pontine changes occurring in these patients [16, 41, 42]. Nonetheless, a similar approach only allows to evaluate some specific features of a condition, possibly neglecting additional meaningful information on other brain regions. This limitation is overcome using voxel-wise analyses, which are known to be highly reproducible, user-independent, and can explore differences over the entire brain without anatomically specific hypotheses [43]. The findings of our study are in line with data in a smaller group of ARSACS patients (n = 9) suggesting the possible occurrence of microstructural changes in some supratentorial and cerebellar areas, as well as through the CST [17]. Similarly, another dMRI study showed the presence of microstructural involvement of different commissural fibers (including forceps minor, forceps major and superior longitudinal fasciculum) in a different small group of patients, suggesting how structural changes might indeed extend beyond motor pathways, also involving the key associative fiber bundles [16]. Thanks to a significantly higher power size and multi-center involvement, our results expand this knowledge, showing the occurrence of multi-faced global neuronal involvement in ARSACS, with loss of neurites integrity, secondary demyelination and overall, a reduction in cellularity, without a specific spatial distribution but with a more prominent involvement of commissural fibers, including the corpus callosum. In particular, the reported loss of axonal and dendritic integrity (often referred together as neurites) is a novel finding in ARSACS. Changes in neurite organization was inferred by the observed changes in NDI, a metric derived from the NODDI model [35] which is known to be able to provide better explanations compared to “classic” dMRI models by increasing specificity for clinically meaningful tissue properties [44]. From a pathophysiological standpoint, this finding could be explained, at least in part, by the evidence of an alteration in mitochondrial transport and dendritic architecture reported in sacsin knockout mice, that seems to precede Purkinje cell death and neurodegeneration [45]. Furthermore, it has also been speculated that sacsin might have a role in the development phase of the brain [16], with a possible imaging counterpart of this abnormal neurodevelopment that could be observed in the misplacement of pontine fibers reported in ARSACS patients[17, 41, 42] as possible sign of aberration of axonal guidance in this condition[46].
Along with modifications in axonal and dendritic integrity, we also found changes in FA, MD, and RD metrics. While FA and MD provide sensitive but non-specific measures of pathology that may be affected by different factors [47], RD changes are supposedly linked to secondary demyelination phenomena. A possible explanation for these observed RD changes can be researched in glial involvement in ARSACS, given the reported accumulation of astroglial intermediate filaments, including glial fibrillary acidic protein (GFAP), in a sacsin knockout rodent model [48]. As astrocytes are known to participate in neuroinflammation [49] and establish numerous interactions with other cells in the nervous system, including neurons [50], we speculate that the observed RD changes (a putative marker of demyelination) might be, therefore, linked to glial involvement, although future research is warranted to elucidate the mechanisms behind this possible link. Interestingly, the voxel-wise analysis showed how commissural fibers, and in particular the corpus callosum, seem to be predominantly involved in ARSACS, also showing a degree of correlation with SARA scores, in absence of correlation with disease duration (thus suggesting that WM changes might represent an early phenomenon in the pathophysiology of damage in these patients). The involvement of this major WM tract is not unexpected, given the reported sacsin expression in this structure [51], which could have a “macroscopical” reflection in the (qualitatively evaluated) reported volume loss of this region [52]. Taken together, our results suggest a prominent role of the corpus callosum in ARSACS, advising for future specific investigations of this major commissural tract, also to evaluate if possible modifications over time of its microstructure could serve as potential biomarker of the disease.
Despite the neuropathologic hallmark of Hereditary Spastic Paraplegias is considered the length-dependent distal axonal degeneration of the CST [53], and that a reduced WM integrity of this structure (along with the brainstem and the frontal lobes) has been anecdotally reported in SPG7 [18, 19], we failed to find any significant microstructural WM change in our group of patients. This suggests that if brain WM microstructure is relatively preserved in SPG7, other neuronal mechanisms of damage might be present, such as a preferential involvement of long axons of peripheral nervous systems. Supporting this speculation, in SPG7 knockout mice, a subset of mitochondria has been selectively reported in the distal region of axons in the spinal cord and the sciatic nerves [54]. Nonetheless, the mechanisms underlying axonal damage require future research.
Although this study has some strengths, such as the large sample size, the acquisition of a standardized harmonized MR protocol across all centers, and the application of advanced analysis models such as NODDI, some limitations should be discussed. In particular, dMRI is not the only technique that can be used to evaluate WM microstructure using MR, with other information that can be obtained by evaluating, for instance, Magnetization Transfer Ratio or Relaxometry. Nonetheless, dMRI is widely accepted as a valuable tool to comprehensively evaluate microstructure in the field of ataxias [5]. Furthermore, a longitudinal evaluation of these variables is also warranted, to understand their possible changes over time, and therefore to fully establish a possible role of dMRI-derived metrics as reliable imaging biomarker in these conditions. Finally, this study was focused on detecting whole brain WM abnormalities, reducing our sensitivity in the detection of possible changes that might occur in specific, small but relevant WM bundles unfeasible to be properly investigates via TBSS (i.e., the superior or inferior cerebellar peduncles) or disregarding microstructural information of other areas of the CNS, such as the spinal cord. Future studies are warranted to investigate the presence of other possible significant WM abnormalities affecting specific WM tracts and the spinal cord in these patients.
Despite the limitations, in this study, we showed how ARSACS, but not SPG7 patients, present a significant, complex and multi-faced involvement of brain WM, with a clinically meaningful widespread loss of axonal and dendritic integrity, secondary demyelination and, overall, a reduction in cellularity and volume.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ARSACS:
-
Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay
- FARS:
-
Friedreich’s Ataxia Rating Scale
- SARA:
-
Scale for the Assessment and Rating of Ataxia
- SPAX:
-
Spastic ataxia
- SPG7:
-
Hereditary Spastic Paraplegia Type 7
- SPRS:
-
Spastic Paraplegia Rating Scale
References
Synofzik M, Schüle R (2017) Overcoming the divide between ataxias and spastic paraplegias: shared phenotypes, genes, and pathways. Mov Disord 32:332–345. https://doi.org/10.1002/mds.26944
Pedroso JL, Vale TC, França Junior MC et al (2021) A diagnostic approach to spastic ataxia syndromes. Cerebellum 21:1073–1084. https://doi.org/10.1007/s12311-021-01345-5
Bereznyakova O, Dupré N (2018) Chapter 12—spastic ataxias. In: Manto M, Huisman TAGM (eds) Handbook of clinical neurology. Elsevier, Amsterdam, pp 191–203
Traschütz A, Adarmes-Gomez AD, Anheim M et al (2023) Autosomal recessive cerebellar ataxias in Europe: frequency, onset, and severity in 677 patients. Mov Disord 38:1109–1112. https://doi.org/10.1002/mds.29397
Öz G, Cocozza S, Henry P-G et al (2023) MR imaging in ataxias: consensus recommendations by the ataxia global initiative working group on MRI biomarkers. Cerebellum. https://doi.org/10.1007/s12311-023-01572-y
Öz G, Harding IH, Krahe J, Reetz K (2020) MR imaging and spectroscopy in degenerative ataxias: toward multimodal, multisite, multistage monitoring of neurodegeneration. Curr Opin Neurol 33:451–461. https://doi.org/10.1097/WCO.0000000000000834
Carreira Figueiredo I, Borgan F, Pasternak O et al (2022) White-matter free-water diffusion MRI in schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacol 47:1413–1420. https://doi.org/10.1038/s41386-022-01272-x
Jelescu IO, Fieremans E (2023) Sensitivity and specificity of diffusion MRI to neuroinflammatory processes. Advances in magnetic resonance technology and applications. Elsevier, Amsterdam, pp 31–50
Lopez-Soley E, Martinez-Heras E, Solana E et al (2023) Diffusion tensor imaging metrics associated with future disability in multiple sclerosis. Sci Rep 13:3565. https://doi.org/10.1038/s41598-023-30502-5
Parikh MN, Chen M, Braimah A et al (2021) Diffusion MRI microstructural abnormalities at term-equivalent age are associated with neurodevelopmental outcomes at 3 years of age in very preterm infants. AJNR Am J Neuroradiol 42:1535–1542. https://doi.org/10.3174/ajnr.A7135
DiPiero M, Rodrigues PG, Gromala A, Dean DC (2022) Applications of advanced diffusion MRI in early brain development: a comprehensive review. Brain Struct Funct 228:367–392. https://doi.org/10.1007/s00429-022-02605-8
Atkinson-Clement C, Pinto S, Eusebio A, Coulon O (2017) Diffusion tensor imaging in Parkinson’s disease: review and meta-analysis. Neuroimage Clin 16:98–110. https://doi.org/10.1016/j.nicl.2017.07.011
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539. https://doi.org/10.1016/j.neuron.2006.08.012
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. https://doi.org/10.1016/j.nurt.2007.05.011
Prodi E, Grisoli M, Panzeri M et al (2013) Supratentorial and pontine MRI abnormalities characterize recessive spastic ataxia of Charlevoix-Saguenay. A comprehensive study of an Italian series. Eur J Neurol 20:138–146. https://doi.org/10.1111/j.1468-1331.2012.03815.x
Oguz KK, Haliloglu G, Temucin C et al (2013) Assessment of whole-brain white matter by DTI in autosomal recessive spastic ataxia of Charlevoix-Saguenay. AJNR Am J Neuroradiol 34:1952–1957. https://doi.org/10.3174/ajnr.A3488
Warnecke T, Duning T, Schirmacher A et al (2010) A novel splice site mutation in the SPG7 gene causing widespread fiber damage in homozygous and heterozygous subjects. Mov Disord 25:413–420. https://doi.org/10.1002/mds.22949
Warnecke T, Duning T, Schwan A et al (2007) A novel form of autosomal recessive hereditary spastic paraplegia caused by a new SPG7 mutation. Neurology 69:368–375. https://doi.org/10.1212/01.wnl.0000266667.91074.fe
PROSPAX—an integrated multimodal progression chart in spastic ataxias. In: PROSPAX. https://www.prospax.net/. Accessed 8 Feb 2024
Schmitz-Hubsch T, Du Montcel ST, Baliko L et al (2006) Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology 66:1717–1720. https://doi.org/10.1212/01.wnl.0000219042.60538.92
Schule R, Holland-Letz T, Klimpe S et al (2006) The spastic paraplegia rating scale (SPRS): a reliable and valid measure of disease severity. Neurology 67:430–434. https://doi.org/10.1212/01.wnl.0000228242.53336.90
Subramony SH, May W, Lynch D et al (2005) Measuring Friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology 64:1261–1262. https://doi.org/10.1212/01.WNL.0000156802.15466.79
Nasreddine ZS, Phillips NA, Bédirian V et al (2005) The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Smith RE, Tournier J-D, Calamante F, Connelly A (2012) Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 62:1924–1938. https://doi.org/10.1016/j.neuroimage.2012.06.005
Tournier J-D, Smith R, Raffelt D et al (2019) MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137. https://doi.org/10.1016/j.neuroimage.2019.116137
Veraart J, Fieremans E, Novikov DS (2016) Diffusion MRI noise mapping using random matrix theory. Magn Reson Med 76:1582–1593. https://doi.org/10.1002/mrm.26059
Veraart J, Novikov DS, Christiaens D et al (2016) Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394–406. https://doi.org/10.1016/j.neuroimage.2016.08.016
Gibbs E, Liu C (2015) Feasibility of imaging tissue electrical conductivity by switching field gradients with MRI. Tomography 1:125–135. https://doi.org/10.18383/j.tom.2015.00142
Horsfield MA (1999) Mapping eddy current induced fields for the correction of diffusion-weighted echo planar images. Magn Reson Imaging 17:1335–1345. https://doi.org/10.1016/S0730-725X(99)00077-6
Yamada H, Abe O, Shizukuishi T et al (2014) Efficacy of distortion correction on diffusion imaging: comparison of FSL Eddy and Eddy_Correct Using 30 and 60 directions diffusion encoding. PLoS ONE 9:e112411. https://doi.org/10.1371/journal.pone.0112411
Fortin J-P, Parker D, Tunç B et al (2017) Harmonization of multi-site diffusion tensor imaging data. Neuroimage 161:149–170. https://doi.org/10.1016/j.neuroimage.2017.08.047
O’Donnell LJ, Westin C-F (2011) An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 22:185–196. https://doi.org/10.1016/j.nec.2010.12.004
Le Bihan D, Mangin J, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. Magn Reson Imaging 13:534–546. https://doi.org/10.1002/jmri.1076
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61:1000–1016. https://doi.org/10.1016/j.neuroimage.2012.03.072
Daducci A, Canales-Rodríguez EJ, Zhang H et al (2015) Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. Neuroimage 105:32–44. https://doi.org/10.1016/j.neuroimage.2014.10.026
Parker CS, Veale T, Bocchetta M et al (2021) Not all voxels are created equal: reducing estimation bias in regional NODDI metrics using tissue-weighted means. Neuroimage 245:118749. https://doi.org/10.1016/j.neuroimage.2021.118749
Andersson JLR, Jenkinson M, Smith S (2007) Non-linear registration aka spatial normalization. FMRIB technial report TR07JA2
Tae W-S, Ham B-J, Pyun S-B et al (2018) Current clinical applications of diffusion-tensor imaging in neurological disorders. J Clin Neurol 14:129. https://doi.org/10.3988/jcn.2018.14.2.129
Lerner A, Mogensen MA, Kim PE et al (2014) Clinical applications of diffusion tensor imaging. World Neurosurg 82:96–109. https://doi.org/10.1016/j.wneu.2013.07.083
Gazulla J, Vela AC, Marín MA et al (2011) Is the ataxia of Charlevoix-Saguenay a developmental disease? Med Hypotheses 77:347–352. https://doi.org/10.1016/j.mehy.2011.05.011
Gazulla J, Benavente I, Vela AC et al (2012) New findings in the ataxia of Charlevoix-Saguenay. J Neurol 259:869–878. https://doi.org/10.1007/s00415-011-6269-5
Lee JE, Chung MK, Lazar M et al (2009) A study of diffusion tensor imaging by tissue-specific, smoothing-compensated voxel-based analysis. Neuroimage 44:870–883. https://doi.org/10.1016/j.neuroimage.2008.09.041
Kamiya K, Hori M, Aoki S (2020) NODDI in clinical research. J Neurosci Methods 346:108908. https://doi.org/10.1016/j.jneumeth.2020.108908
Girard M, Larivière R, Parfitt DA et al (2012) Mitochondrial dysfunction and Purkinje cell loss in autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS). Proc Natl Acad Sci USA 109:1661–1666. https://doi.org/10.1073/pnas.1113166109
Engle EC (2010) Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol 2:a001784–a001784. https://doi.org/10.1101/cshperspect.a001784
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265. https://doi.org/10.3978/j.issn.2223-4292.2012.12.05
Murtinheira F, Migueis M, Letra-Vilela R et al (2022) Sacsin deletion induces aggregation of glial intermediate filaments. Cells 11:299. https://doi.org/10.3390/cells11020299
Giovannoni F, Quintana FJ (2020) The role of astrocytes in CNS inflammation. Trends Immunol 41:805–819. https://doi.org/10.1016/j.it.2020.07.007
Abdelhak A, Foschi M, Abu-Rumeileh S et al (2022) Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol 18:158–172. https://doi.org/10.1038/s41582-021-00616-3
Larivière R, Sgarioto N, Márquez BT et al (2019) Sacs R272C missense homozygous mice develop an ataxia phenotype. Mol Brain 12:19. https://doi.org/10.1186/s13041-019-0438-3
Cocozza S, Pontillo G, De Michele G et al (2021) Conventional MRI findings in hereditary degenerative ataxias: a pictorial review. Neuroradiology 63:983–999. https://doi.org/10.1007/s00234-021-02682-2
Lo Giudice T, Lombardi F, Santorelli FM et al (2014) Hereditary spastic paraplegia: clinical-genetic characteristics and evolving molecular mechanisms. Exp Neurol 261:518–539. https://doi.org/10.1016/j.expneurol.2014.06.011
Ferreirinha F, Quattrini A, Pirozzi M et al (2004) Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J Clin Invest 113:231–242. https://doi.org/10.1172/JCI200420138
Acknowledgements
This project was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) No. 441409627, as part of the PROSPAX consortium under the frame of EJP RD, the European Joint Programme on Rare Diseases, under the EJP RD COFUND-EJP No. 825575. FMS is partially supported by the Italian Ministry of Health, Ricerca Corrente 2024.
Collaborators for the PROSPAX Consortium
Graziella Donatelli (graziella_donatelli@hotmail.com—Neuroradiology Unit, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy—Imago7 Research Foundation, Pisa, Italy); Giovanna De Michele (giodemic@gmail.com—Department of Neurosciences and Reproductive and Odontostomatological Sciences, University of Naples “Federico II”, Naples, Italy); Thomas M. Ernst (thomas.ernst@uk-essen.de—Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Essen University Hospital, Essen, Germany); Stephan Klebe (stephan.klebe@uk-essen.de—Department of Neurology, University Hospital Essen, Essen, Germany); Ilana Leppert (ilana.leppert@mcgill.ca—McConnell Brain Imaging Center, Montreal Neurological Institute, McGill University); Ivana Ricca (ivanaricca.fsm@gmail.com—Department of Molecular Medicine, IRCCS Stella Maris Foundation, Pisa, Italy); Sara Satolli (sara.satolli@gmail.com—Department of Molecular Medicine, IRCCS Stella Maris Foundation, Pisa, Italy); Andreas Traschütz (andreas.traschuetz@uni-tuebingen.de—Division Translational Genomics of Neurodegenerative Diseases, Center for Neurology and Hertie Institute for Clinical Brain Research, University of Tübingen, Tübingen, Germany; German Center for Neurodegenerative Diseases (DZNE), Tübingen, Germany).
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Consortia
Contributions
A.S.: drafting a significant portion of the manuscript or figures, acquisition of data. I.G., G.M., M.B., S.B., S.S.: analysis of data, drafting a significant portion of the manuscript or figures. B.B., C.K., B.B., R.L.P., B.P.v.d.W., M.C., D.T., PROSPAX Consortium: acquisition of data, drafting a significant portion of the manuscript or figures. A.D., R.S., M.S., F.M.S., S.C.: conception and design of the study, acquisition of data, drafting a significant portion of the manuscript or figures.
Corresponding author
Ethics declarations
Conflicts of interest
SC have received fees for Advisory Board by Amicus. All other co-authors have no possible financial conflict of interest to declare.
Ethical approval
The study was approved by local Ethics Committee of each center and written informed consent was obtained from each participant.
Additional information
Collaborators of the PROSPAX Consortium are listed in “Acknowledgements” section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scaravilli, A., Gabusi, I., Mari, G. et al. An MRI evaluation of white matter involvement in paradigmatic forms of spastic ataxia: results from the multi-center PROSPAX study. J Neurol (2024). https://doi.org/10.1007/s00415-024-12505-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12505-y