Abstract
Background
This study was an introduction to the Swedish ALSrisc Study and explored the association of lifestyle and medical conditions, with risk and progression of amyotrophic lateral sclerosis (ALS).
Methods
We included 265 newly diagnosed ALS patients during 2016–2022 in Stockholm and 207 ALS-free siblings and partners of the patients as controls. Information on body mass index (BMI), smoking, and history of head injuries, diabetes mellitus, hypercholesterolemia, and hypertension was obtained through the Euro-MOTOR questionnaire at recruitment. Patients were followed from diagnosis until death, invasive ventilation, or November 30, 2022.
Results
Higher BMI at recruitment was associated with lower risk for ALS (OR 0.89, 95%CI 0.83–0.95), especially among those diagnosed after 65 years. One unit increase in the average BMI during the 3 decades before diagnosis was associated with a lower risk for ALS (OR 0.94, 95%CI 0.89–0.99). Diabetes was associated with lower risk of ALS (OR 0.38, 95%CI 0.16–0.90), while hypercholesterolemia was associated with higher risk of ALS (OR 2.10, 95%CI 1.13–3.90). Higher BMI at diagnosis was associated with lower risk of death (HR 0.91, 95%CI 0.84–0.98), while the highest level of smoking exposure (in pack-years) (HR 1.90, 95%CI 1.20–3.00), hypercholesterolemia (HR 1.84, 95%CI 1.06–3.19), and hypertension (HR 1.76, 95%CI 1.03–3.01) were associated with higher risk of death, following ALS diagnosis.
Conclusions
Higher BMI and diabetes were associated with lower risk of ALS. Higher BMI was associated with lower risk of death, whereas smoking (especially in high pack-years), hypercholesterolemia, and hypertension were associated with higher risk of death after ALS diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a relatively rare neurodegenerative disease with a poor prognosis [1]. Several mutations following either a monogenic, oligogenic, or polygenic inheritance have been associated with ALS, indicating a genetic component in the etiology of ALS [1]. Nevertheless, the heritability of the disease is estimated to be around 50% [2], suggesting that other factors, epigenetic or environmental, also contribute to the pathophysiology of ALS. The cause of ALS is hypothesized to involve genetic triggers of varying severity and their interaction with environmental risk factors through epigenomic changes at various time points during the lifespan [3]. The time of disease onset might depend on the mutation’s biological significance and the degree of exposure to environmental factors over time [4].
The most common monogenic mutation linked with ALS, especially in populations of European ancestry, is the repeat expansion of the GGGGCC hexanucleotide in the first intron of the C9orf72 gene [5]. Repeats of 24 or more hexanucleotides are considered pathogenic, leading to earlier diagnosis of ALS [6, 7]. Nonetheless, monogenic ALS mutations may still exhibit incomplete penetrance or delayed onset [8], as exposure to certain environmental factors might modify the risk the mutations confer [8]. Many environmental risk factors have been studied in relation to ALS [9, 10], with some of the associations potentially varying by age, sex, and genetic factors [11,12,13,14,15].
The aim of this study was first to introduce the Swedish ALSrisc Study and then to explore the role of body mass index (BMI), smoking, and history of head injuries, hypercholesterolemia, diabetes mellitus, or hypertension on the risk and prognosis of ALS, focusing on the potential modifying effect of age, sex, and the mutation status of C9orf72.
Methods
ALSrisc study
The ALSrisc Study (“Biomarkers and Risk Factors for Amyotrophic Lateral Sclerosis”) is an ongoing case–control study that started in 2016 in Stockholm, Sweden, with the overall goal of improving the understanding of ALS disease mechanisms by studying how specific biomarkers, lifestyle, and environmental factors are related to the risk and prognosis of ALS (see Fig. 1 for a graphical summary of the ALSrisc Study). Cases of the ALSrisc Study include all patients with a new diagnosis of ALS, progressive spinal muscular atrophy (PSMA), primary lateral sclerosis (PLS), or any other motor neuron diseases (MNDs) since January 2016. The patients are all diagnosed at the ALS Clinical Research Center, Karolinska University Hospital, the only tertiary center for ALS patients in the Stockholm region with a population of over 2 million. All patients with ALS met the revised El Escorial criteria for clinically definite, probable, or possible ALS. Furthermore, the ALSrisc Study includes two control groups, namely ALS-free full siblings and spouses of the cases. Cases, sibling controls, and spouse controls are enrolled at the time of diagnosis or shortly thereafter. Individuals not speaking Swedish and individuals who migrated out of the Stockholm region after recruitment (without complete follow-up) are excluded from the study. Figure 2 shows the recruitment rate of ALS patients by year in the ALSrisc Study.
Overview of the ALSrisc Study. The central part of the figure illustrates the recruitment of patients and their relative controls (siblings and spouse) at the time of diagnosis of the patient or shortly thereafter. The circle represents the follow-up of the patients from diagnosis until death, initiation of invasive ventilation, or withdrawal from the study. A diverse set of information is collected, through linkage to the Swedish Motor Neuron Disease (MND) Quality Registry and electronic medical records, self-reported data from questionnaires, biological specimens, and laboratory investigations at recruitment or during follow-up (for patients only and at specific time intervals). The letter B indicates that information is collected at baseline (recruitment). The numbers “6” and “12” indicate that information is collected every 6 or 12 months. The letter C indicates that information is collected continuously. Blue indicates that information is collected only from ALS patients, whereas green indicates that information is collected from both patients and controls
Data collected in the study come from three major sources, namely medical records data, questionnaire data, and biological samples. Clinical characteristics of the ALS patients are collected from medical records, including time and site of disease onset, disease progression rate, spreading pattern of paresis, ALS Functional Rating Score-revised (ALSFRS-R), neurophysiology findings, medications, cognitive impairment including frontotemporal dementia (FTD), etc. Screening for a panel of 197 genes (e.g., SOD1, TDP-43, and C9orf72) is performed as clinical routine for ALS patients. In addition, patients are asked to participate in further examinations, including neuroimaging and cardiac examinations.
All patients included in the ALSrisc Study are automatically followed up through the Swedish MND Quality Registry [16]. Since 2015, this registry has continuously collected information on clinical measures, biological samples, and quality-of-life outcomes from the majority of the MND patients in Sweden at the time of diagnosis and each follow-up clinic visit every 3 months after diagnosis. Since 2017, this registry has collected information on nearly all MND patients in Stockholm. Through a structured interview using a questionnaire modified from a large European consortium for ALS “Euro-MOTOR”, we also collect self-reported information on previous environmental exposures (e.g., housing, employment, and military service), lifestyle factors (e.g., smoking, alcohol use, and physical activity), and medical history from the cases and controls [17]. We also use Food Frequency Questionnaires to collect information on past and current dietary habits from the cases and controls.
For ALS patients, we collect information at recruitment as well as every 6 months thereafter, on different measurements of mental health including ADI-12 (ALS Depression Inventory-12), HAD (Hospital Anxiety and Depression Scale), and DAS (Depression, Anxiety and Stress scale), cognitive function including MoCA (Montreal Cognitive Assessment) and ECAS (Edinburgh Cognitive and Behavioural ALS Screen ), quality of life including ESS (Epworth Sleepiness Scale), CIS20R (Checklist Individual Strength, Radbound University), ACSA (Amnestic Comparative Self-Assessment), EQ-5D-5L (EuroQoL 5-Dimensions 5-Levels), SF-12 (12-item Short Form Survey), MNDCS (Motor Neuron Disease Coping Scale), as well as Coping-AMS (Achievement Motives Scale), and the Lifestyle and Nutrition Survey.
All participants donate blood (stored as whole blood, plasma, and serum), feces, saliva, hair, and nails at enrollment. Feces, saliva, and blood samples are stored frozen at the Karolinska Institutet Biobank at −80 °C, whereas hair and nail samples are stored at room temperature. In addition, we collect cerebrospinal fluid (CSF) from the cases. Cases also donate these biological samples annually during the follow-up. To date, the ALSrisc Study has built up a large biobank including data and biospecimens collected from over 400 ALS patients and over 200 controls. The recruited ALS patients are largely representative of all ALS patients diagnosed during the same period in Stockholm, according to the Swedish MND Quality Registry (Table 1).
Study material
In the present study, we included 265 patients with newly diagnosed ALS from March 2016 to May 2022 recruited to the ALSrisc Study and followed these patients from the date of diagnosis until the date of death, initiation of invasive ventilation, or the end of follow-up (November 30, 2022), whichever came first. The site of disease onset was classified as bulbar, spinal, or other (including respiratory and frontal lobe onset). The time to diagnosis was calculated as the time interval between the onset of muscle paresis and the diagnosis of ALS. The functional decline was calculated using the ALSFRS-R measured at diagnosis and during follow-up. The disease progression rate at diagnosis was calculated by the following formula: (48–ALSFRS-R score at diagnosis)/diagnostic delay (in months). Information on C9orf72 mutation status was procured from the genetic panel mentioned above.
We included 207 controls (79 siblings and 128 partners of the ALS patients) in the present study. None of these siblings tested positive for C9orf72 mutation and remained asymptomatic during the study. In addition to age and sex, self-reported information on BMI, smoking, and history of head injuries, diabetes mellitus, hypercholesterolemia, or hypertension was collected at recruitment for both cases and controls. BMI was estimated as the ratio of the current reported weight (kilograms) and height (m2) at recruitment, as well as for each decade before recruitment since the age of 20. Smoking status was classified as current, former, or never. We calculated the pack-years of smoking by multiplying the number of cigarettes smoked daily by the years of smoking. Study participants were then categorized into four groups: never smoked, smoked < 7 pack-years, smoked 7–16 pack-years, or smoked > 16 pack-years. The latter three groups were classified based on the tertile distribution of pack-years among the participants who smoked. Information on past medical history was collected to establish a previous diagnosis of diabetes mellitus, hypercholesterolemia, and hypertension. Self-reported information on the use of medications for the treatment of hypercholesterolemia and hypertension was used as an additional source to ascertain history of hypercholesterolemia and hypertension.
Statistical analysis
We first used unconditional logistic regression models to calculate the odds ratio (OR) of ALS with 95% confidence interval (95% CI) in relation to BMI, smoking, and history of head injuries, diabetes mellitus, hypercholesterolemia, or hypertension, by comparing cases and controls. In the analysis of smoking, we examined the risk of ALS in relation to smoking status (never, former, or current) as well as by different pack-years of smoking (never smoked, or < 7, 7–16, or > 16 pack-years). Additionally, we assessed the non-linear association between BMI, modeled with restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles, and ALS risk. As a secondary analysis, we used conditional logistic regression models with the family identifier as stratum to account for potential familial confounding due to shared early life environmental and genetic factors (between siblings) and adult-life environmental factors (between partners). In both analyses, we adjusted for age at recruitment and sex. For BMI during the 3 decades before recruitment, we also used generalized estimating equations (GEE) with independent correlation structure and robust standard errors to calculate the association of BMI reported for every decade until recruitment with the risk for ALS. The GEE model was adjusted for age and sex. Finally, we used the method of locally weighted scatterplot smoothing (LOWESS) to plot the mean BMI, separately for cases and controls, as well as for cases of different C9orf72 mutation status, during each decade before recruitment.
Then, to study the association of BMI, smoking, and history of head injuries, diabetes mellitus, hypercholesterolemia, or hypertension with the time-varying ALSFRS-R score and the risk of death, a joint model that combines a linear mixed-effects model for all the repeated measurements of ALSFRS-R score and a survival model of censored outcomes was used. In the longitudinal component of the model, we considered a random intercept, a random slope, and an unstructured covariance matrix, while for the survival model, we considered a Royston–Parmar proportional hazards survival model where the baseline log hazard function was modeled with restricted cubic splines with four knots at the 5th, 35th, 65th, and 95th percentiles. The proportional hazards assumption was tested using scaled Schoenfeld residuals, and interactions with time were introduced for any variables violating this assumption. The attained age was used as the underlying time scale, and the date of birth was used as the time origin. The longitudinal and the survival components were linked through the shared random effects. The model was adjusted for age at diagnosis, sex, site of onset, diagnostic delay, and BMI at diagnosis. The latter was not included in models where the main independent variable was BMI. In secondary analyses, we also performed a Cox model and a linear mixed model to assess the associations between these factors and risk of death or longitudinal changes of ALSFRS-R after a diagnosis of ALS, respectively, after adjusting for age at diagnosis, sex, site of onset, diagnostic delay, and BMI at diagnosis. The survival model was additionally adjusted for ALSFRS-R and progression rate at the time of diagnosis.
Statistical analyses were performed using STATA (Release 16. College Station, TX: StataCorp LLC.). A predefined significance level of 5% was used for all the analyses. Reporting of the present study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (Supplementary File 1). The missingness level was low (5–10%) in variables with missing values; thus, we performed complete case analyses. Figure 1 was created using BioRender.com.
Standard protocol approval, registration, and patient consent
The study was approved by the Swedish Ethical Review Authority (DNRs: 2014–1815-31–4, 2018–1605-31, and 2021–06397-02). Oral and written informed consent was collected from all study participants.
Results
In the present study, 117 cases (44.3%) were women, whereas 116 controls were women (56%). The mean (SD) age at recruitment was 65.6 (10.6) for cases and 63.7 (10.7) for controls (Table 2, Supplementary Table 1). Among the cases, the mean (SD) ALSFRS-R score at diagnosis was 37.1 (7.6). Spinal onset was observed in 62.7% of the cases, and bulbar onset in 30.4%. The median (IQR) diagnostic delay was 13.4 (8.6–20.9) months.
Cases versus controls
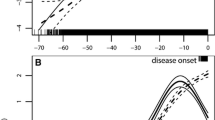
In the unconditional logistic regression, one-unit increase in BMI at recruitment was associated with 10% lower odds for ALS (OR 0.90, 95%CI 0.85–0.96) (Table 3). The analysis by age at recruitment showed that a statistically significant association was only observed among individuals 65 years or older (Fig. 3). At the time of recruitment, compared to a BMI of 21, a lower BMI was associated with higher odds of ALS, whereas a higher BMI was associated with lower odds (Fig. 4). The conditional logistic regression models yielded similar results, showing a slightly stronger association for BMI at recruitment, a positive association between hypercholesterolemia and ALS (OR 2.10, 95%CI 1.13–3.90), and an inverse association between diabetes mellitus and ALS (OR 0.38, 95%CI 0.16–0.89). After adjusting additionally for the earliest available BMI measurement which was on average 42.6 ± 11.7 years before diagnosis, we found similar associations for diabetes mellitus (OR 0.35, 95%CI 0.15–0.85) and hypercholesterolemia (OR 2.21, 95%CI 1.14–4.26). Although no association was noted between BMI during the three different decades before recruitment and the risk of ALS, we found an increasing BMI in both cases and controls by time to recruitment (Fig. 5). Although cases had a slightly higher BMI than controls earlier in life, they experienced a slower increase in BMI along with aging, resulting in lower BMI levels during the last decade before diagnosis. This trend was similar when comparing ALS patients without the C9orf72 mutation (C9- cases) to controls. The difference was, however, negligible between ALS patients with the C9orf72 mutation (C9 + cases) and controls. Table 4 shows the association of one-unit increase in BMI for every decade during the 3 decades before recruitment with the risk for ALS. Over time, one-unit increase in BMI was associated with 6% lower odds for ALS (OR 0.94, 95%CI 0.89–0.99). This association did not differ greatly by sex or C9 status.
Odds ratio (OR) with 95% confidence interval (CI) for ALS per 1 kg/m2 increase in BMI measured at recruitment, adjusted for sex—stratified analysis by age at recruitment. Odds ratio (circle) with 95% confidence interval (line). The absolute numbers of cases and controls are shown in the bottom of the figure
Odds ratio (OR) and 95% confidence interval (CI) for ALS in relation to BMI measured at recruitment, adjusted for sex analysis using BMI as a continuous variable. Odds ratio (solid line) with 95% confidence interval (dashed lines) for ALS in relation to BMI (Kg/m2) modeled with restricted cubic splines with three knots placed at a BMI of 20.7, 24.2, and 29.9, respectively. Estimates are derived from unconditional logistic regression with linear and spline terms for BMI and a BMI of 21 kg/m2 as the reference value
Analyses among cases
During a median (IQR) follow-up of 1.55 (0.95–2.45) years, we observed a total of 164 deaths or initiation of invasive ventilation among the 265 ALS patients. Table 5 shows the association of the studied factors with the risk of death following an ALS diagnosis, after accounting for the repeated measurements of ALSFRS-R after ALS diagnosis. Patients with a higher BMI at diagnosis had a lower risk of death (HR 0.91, 95%CI 0.84–0.98), while a history of hypercholesterolemia (HR 1.84, 95%CI 1.06–3.19) or hypertension (HR 1.76, 95%CI 1.03–3.01) was associated with a higher risk of death after ALS diagnosis. Current smoking (HR 2.28; 95%CI 0.91–5.71) and the highest level of smoking in pack-years (HR 1.90, 95%CI 1.20–3.00) were both associated with a higher risk of death, although the association was only statistically significant for the latter. No association was found for history of head injuries or diabetes mellitus. Similar estimates were found when studying the risk of death without accounting for the longitudinal change of ALSFRS-R (Supplementary Table 2). In the analysis of repeated measurements for ALSFRS-R alone, ALS patients with the highest level of smoking in pack-years (β −3.81, 95%CI −7.24 to −0.38) or a history of hypertension (β −4.55, 95%CI −7.22 to −1.89) or hypercholesterolemia (β −2.86, 95%CI −5.75 to 0.02) had a faster decline in ALSFRS-R compared with other patients (Supplementary Table 3).
Discussion
We investigated BMI, smoking, and history of head injuries, hypercholesterolemia, diabetes mellitus, or hypertension in relation to the risk of ALS in the Swedish ALSrisc Study. Among the ALS patients, we explored the association of these factors with the risk of death and functional decline after diagnosis. In brief, we found that ALS patients, primarily those above 65, had a lower BMI at the time of diagnosis, compared to controls. We further showed that a one-unit increase in the average BMI during the 30 years before diagnosis was associated with a 6% lower risk for ALS. Diabetes was also associated with a lower risk of ALS. A higher BMI at the time of diagnosis was associated with a lower risk of death, whereas smoking (especially in higher dose) and a history of hypertension or hypercholesterolemia were associated with a higher risk of death following an ALS diagnosis.
Hypermetabolism and weight loss have been previously shown among ALS patients [18]. Energy imbalance, altered thermogenesis, and gut microbiome alterations have been suggested as some of the mechanisms involved [18, 19]. Loss of appetite and dysphagia are two other factors suggested to contribute to weight loss among ALS patients [20]. Our findings on BMI are consistent with those of previous studies. A lower BMI has been associated with a higher risk for ALS [7, 11, 21,22,23]. We additionally showed that, during the decades before diagnosis, the rate of BMI increase tended to be different between ALS patients and controls. Although ALS patients had a higher BMI compared to controls earlier in life, they presented with lower BMI during the 10 years before diagnosis, in accordance with the previous studies [22, 23]. This finding was sustained after adjusting for age and sex. A study by Westeneng et al. suggested that the median BMI was higher among C9- patients but lower among C9 + patients, compared to controls, during several decades before diagnosis [7]. We showed a similar trend for C9- patients compared to controls as in the entire sample; however, the difference was less tangible between C9 + patients and controls. A lower BMI at diagnosis [21, 24,25,26] and an increased weight loss before diagnosis [27, 28] have been previously related to a higher risk of death after ALS diagnosis. Other studies that considered ALSFRS-R score as a measure of disease progression have also shown that a lower BMI is associated with a faster functional decline [29]. In line with prior studies, we showed that a higher BMI was associated with a lower risk of death among ALS patients, after accounting for the functional decline over time.
Besides BMI, another indication for metabolic dysregulation is altered lipid metabolism. The association observed for a history of hypercholesterolemia and a higher risk of ALS in this study is consistent with Mendelian randomization studies suggesting a causal positive association between total cholesterol and low-density lipoprotein (LDL) and ALS risk [30, 31] as well as with observational studies indicating a higher level of total cholesterol among ALS cases than controls [32]. Previous research by our group and others has found a null or inverse association between statin use and ALS risk [33, 34]. Thus, even if we assume that most participants reporting hypercholesterolemia had taken cholesterol-lowering medications, including statins, the observed association of hypercholesterolemia with ALS risk might not be entirely explained by medication use. The link between hypercholesterolemia and ALS is believably complex. Long-term hypercholesterolemia might be protective against ALS as lipids can serve as an alternative energy source for neurons, counteracting the neurodegenerative effects of energy deficit [35]. On the other hand, ALS-related pathologies might lead to alterations in cholesterol metabolism, potentially leading to the development of hypercholesterolemia. For instance, TDP-43 was previously shown to modify the expression of the sterol regulatory element binding transcription factor 2 (SREBF2) protein which is involved in the transcriptional control of cholesterol metabolism [36]. Further studies are therefore needed to better understand whether the higher-than-expected prevalence of hypercholesterolemia among patients with ALS is an upstream or secondary event of ALS. Our finding of an increased mortality among ALS patients with a history of hypercholesterolemia contrasts, on the other hand, with a prior study suggesting that higher lipoprotein levels measured at the time of diagnosis conveyed a lower risk of death after ALS diagnosis [37]. However, it is worth noting that we studied the history of hypercholesterolemia, whereas the previous study examined levels of lipid fractions, which were mostly within the normal range.
We found an inverse association between a history of diabetes mellitus and ALS risk, which is in line with previous research showing a protective role of type 2 diabetes in the risk of ALS [38,39,40]. Based on the information we had available from the Euro-MOTOR questionnaire we could not explicitly differentiate diabetes mellitus type 2 from type 1. Considering that diabetes mellitus type 1 has been shown to have a positive association with the risk of ALS, we expect that the misclassification of some cases of diabetes mellitus type 1 as type 2 would most likely have underestimated the real inverse association between diabetes type 2 and risk of ALS [38, 41]. Previous studies have suggested several pathways potentially underlying the observed inverse association between diabetes mellitus type 2 and ALS risk; for instance, a high circulating level of glucose and a higher energy balance can potentially counteract the energy deficit of ALS related to aberrant aggregations of proteins in neurons [35]. The observed association might also be in part attributed to the use of antidiabetic medication [42]. Nevertheless, the effect of antidiabetic medication was not possible to investigate as the Euro-MOTOR questionnaire does not ask for such information.
In our study, we found hypertension to be associated with both a higher risk of death as well as a faster functional decline in ALS patients. In line with our results, history of hypertension was previously linked to a higher risk of death among ALS patients [43, 44]. In contrast to our study, however, another study showed an inverse association of hypertension with ALS risk [45]. The contrasting findings might be because the increment in systolic and diastolic blood pressure might eventually have opposite effects on the risk for ALS; namely, systolic blood pressure might be a risk factor, whereas diastolic blood pressure might be a protective factor, for ALS [46]. In a sample of healthy individuals over 60 years, kidney function was found to negatively correlate with neurofilament light chain (NfL) [47], partially explaining the link between hypertension and a poorer prognosis after ALS diagnosis. A higher stage of chronic kidney disease and a lower concentration of cystatin C-based-eGFR have also been associated with ALS risk [48], indicating a potential explanatory mechanism for the link between hypertension and ALS.
Smoking has been considered for a long time as a potential risk factor for ALS. The exposure to oxidative stress and neurotoxic chemicals might provide a plausible biological explanation for this association [14]. Some observational studies showed a positive association for current smoking but no association for former smoking [7, 14, 15], while Mendelian Randomization studies have provided evidence for a causal link between smoking and risk for ALS [49]. Nevertheless, other studies, including a meta-analysis, suggested that smoking is not associated with the risk for ALS [11, 12] or risk of death after ALS diagnosis [13]. Our study did not find an association between current smoking and risk of ALS. Compared to controls, ALS patients might be more likely to quit smoking since the onset of symptoms, biasing therefore the association between smoking and ALS risk toward null. However, our study showed that current smoking and high level of smoking exposure (in pack-years) were associated with increased mortality risk among ALS patients, although the association was only statistically significant in the latter, in agreement with a previous study [50].
Head injury has been suggested as a risk factor for several neurodegenerative diseases as head trauma may for instance disrupt the permeability of the blood–brain barrier [51]. Additional indications for a role of head trauma in neurodegeneration include the TDP-43 depositions found in animal models following head trauma and in cases of chronic traumatic encephalopathy [51, 52]. A recent meta-analysis by Liu et al. [51] found a positive association between a history of head trauma and risk for ALS. Both severe and less severe head trauma were associated with an increased risk for ALS. The risk was three times as high for injuries occurring less than a year before diagnosis, indicating potentially reverse causation, considering that ALS is a disease with at least 1 year of diagnostic delay. The null result of the present study might also be partially attributed to a lack of statistical power to identify an association between a history of head injuries and the risk or prognosis of ALS. Further, as we had little information on the characteristics of head injuries, such as neuroimaging findings, we cannot preclude the possibility that head injuries of specific characteristics might still be related to the risk and prognosis of ALS.
The strengths of the present study lie in the inclusion of only incident ALS patients and the complete follow-up for all patients, alleviating concerns on selection bias due to recruitment of prevalent cases (i.e., patients with better survival than average) and loss to follow-up. A novelty of our study is the use of a study design that alleviates concern on familial confounding through analyzing discordant pairs of siblings or partners, because siblings and spouses share genetic or non-genetic factors that might be common causes of the studied lifestyle and medical conditions and ALS. The simultaneous investigation of the risk and prognosis of ALS, together with the exploration of the possible interplay between lifestyle and medical conditions and age, sex, and C9orf72 mutation status, constitute two other novelties of the present study, compared to the existing literature. Finally, employing the joint model accounting for the longitudinal changes of ALSFRS-R, facilitated a more robust prognostic analysis [53].
Still, this study has several limitations. First, the information on exposure status was self-reported and obtained retrospectively. Thus, some degree of recall bias might exist for cases and the controls. For BMI during the decades before recruitment, it is possible that both cases and controls did not remember accurately, introducing some degree of non-differential misclassification. If this is the case, the effect we observed might have underestimated the real effect of BMI on ALS risk. Second, we had information on diabetes mellitus, hypercholesterolemia, and hypertension through self-reported diagnosis and on hypercholesterolemia and hypertension through also medication use; however, we had little information on how well the conditions were controlled. As a result, it remains to be examined whether the results would differ between conditions that are well controlled and conditions that are poorly controlled. Third, the sample size was not large enough to study these associations separately for sibling and partner controls. Further, we did not measure the extent of the C9orf72 hexanucleotide extension for the ALS patients; as a result, it remains to be assessed whether the number of repeats could modify some of the noted associations in the study. Finally, although we studied a rather extensive list of factors potentially related to the risk and prognosis of ALS, we focused on the individual roles of these factors. Future studies are encouraged to examine the potentially synergistic effects of different risk factors. For instance, to better understand the link between BMI and ALS, other variables related to energy intake and expenditure, including dietary factors, physical activity, and the composition and functionality of gut microbiota should also be taken into account.
Conclusion
Based on the Swedish ALSrisc Study, a higher BMI and previous diabetes mellitus were associated with a lower risk of ALS. In comparison, hypercholesterolemia was associated with a higher risk of ALS. A higher BMI was also associated with a lower risk of death, whereas smoking (especially in high pack-years), hypercholesterolemia, and hypertension were associated with a higher risk of death, after ALS diagnosis. Further investigation is warranted to understand whether the lower BMI, lower prevalence of diabetes mellitus, and higher prevalence of hypercholesterolemia among ALS patients are risk factors for ALS or secondary to ALS. The findings of a higher risk of death among ALS patients with smoking (especially heavy smoking), hypercholesterolemia, and hypertension underscore on the other hand the importance of targeting these conditions, with the aim of improving the survival of ALS patients.
Data availability
Researchers can apply for access to data from the present study for well-defined research questions that are in line with the overall research agenda of the ALSrisc Study. For more information, please visit https://ki.se/en/cns/pre-clinical-research.
References
Goutman SA, Hardiman O, Al-Chalabi A et al (2022) Emerging insights into the complex genetics and pathophysiology of amyotrophic lateral sclerosis. Lancet Neurol 21:465–479
Ryan M, Heverin M, McLaughlin RL, Hardiman O (2019) Lifetime Risk and Heritability of Amyotrophic Lateral Sclerosis. JAMA Neurol 76:1367–1374
Chiò A, Mazzini L, D’Alfonso S et al (2018) The multistep hypothesis of ALS revisited: The role of genetic mutations. Neurology 91:e635–e642
Al-Chalabi A, Hardiman O (2013) The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 9:617–628
Zou ZY, Zhou ZR, Che CH, Liu CY, He RL, Huang HP (2017) Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 88:540–549
Iacoangeli A, Al Khleifat A, Jones AR et al (2019) C9orf72 intermediate expansions of 24–30 repeats are associated with ALS. Acta Neuropathol Commun 7:115
Westeneng HJ, van Veenhuijzen K, van der Spek RA et al (2021) Associations between lifestyle and amyotrophic lateral sclerosis stratified by C9orf72 genotype: a longitudinal, population-based, case-control study. Lancet Neurol 20:373–384
Goutman SA, Hardiman O, Al-Chalabi A et al (2022) Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol 21:480–493
Ingre C, Roos PM, Piehl F, Kamel F, Fang F (2015) Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol 7:181–193
Duan QQ, Jiang Z, Su WM et al (2023) Risk factors of amyotrophic lateral sclerosis: a global meta-summary. Front Neurosci 17:1177431
Nakken O, Meyer HE, Stigum H, Holmøy T (2019) High BMI is associated with low ALS risk: A population-based study. Neurology 93:e424–e432
Alonso A, Logroscino G, Hernán MA (2010) Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 81:1249–1252
Batty GD, Gale CR (2021) Pre-Morbid Risk Factors for Amyotrophic Lateral Sclerosis: Prospective Cohort Study. Clin Epidemiol 13:941–947
Opie-Martin S, Jones A, Iacoangeli A et al (2020) UK case control study of smoking and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 21:222–227
Peters S, Visser AE, D’Ovidio F et al (2020) Effect modification of the association between total cigarette smoking and ALS risk by intensity, duration and time-since-quitting: Euro-MOTOR. J Neurol Neurosurg Psychiatry 91:33–39
Longinetti E, Regodón Wallin A, Samuelsson K et al (2018) The Swedish motor neuron disease quality registry. Amyotroph Lateral Scler Frontotemporal Degener 19:528–537
D’Ovidio F, Rooney JPK, Visser AE et al (2017) Critical issues in ALS case-control studies: the case of the Euro-MOTOR study. Amyotr Lateral Scler Frontotemp Degener 18:411–418
Burg T, Van Den Bosch L (2023) Abnormal energy metabolism in ALS: a key player? Curr Opin Neurol 36:338–345
Bolborea M, Vercruysse P, Daria T et al (2023) Loss of hypothalamic MCH decreases food intake in amyotrophic lateral sclerosis. Acta Neuropathol 145:773–791
Ahmed RM, Caga J, Devenney E et al (2016) Cognition and eating behavior in amyotrophic lateral sclerosis: effect on survival. J Neurol 263:1593–1603
Mariosa D, Beard JD, Umbach DM et al (2017) Body Mass Index and Amyotrophic Lateral Sclerosis: A Study of US Military Veterans. Am J Epidemiol 185:362–371
Goutman SA, Boss J, Iyer G et al (2023) Body mass index associates with amyotrophic lateral sclerosis survival and metabolomic profiles. Muscle Nerve 67:208–216
Peter RS, Rosenbohm A, Dupuis L et al (2017) Life course body mass index and risk and prognosis of amyotrophic lateral sclerosis: results from the ALS registry Swabia. Eur J Epidemiol 32:901–908
Calvo A, Moglia C, Lunetta C et al (2017) Factors predicting survival in ALS: a multicenter Italian study. J Neurol 264:54–63
Shimizu T, Nagaoka U, Nakayama Y et al (2012) Reduction rate of body mass index predicts prognosis for survival in amyotrophic lateral sclerosis: a multicenter study in Japan. Amyotr Lateral Scler 13:363–366
Janse Van Mantgem MR, Van Eijk RPA, Van Der Burgh HK et al (2020) Prognostic value of weight loss in patients with amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 91:867–875
Marin B, Arcuti S, Jesus P et al (2016) Population-Based Evidence that Survival in Amyotrophic Lateral Sclerosis is Related to Weight Loss at Diagnosis. Neurodegener Dis 16:225–234
Wolf J, Safer A, Wöhrle JC et al (2014) Factors predicting one-year mortality in amyotrophic lateral sclerosis patients–data from a population-based registry. BMC Neurol 14:197
Hannaford A, Byth K, Pavey N et al (2023) Clinical and neurophysiological biomarkers of disease progression in amyotrophic lateral sclerosis. Muscle Nerve 67:17–24
Xia K, Klose V, Högel J et al (2023) Lipids and amyotrophic lateral sclerosis: A two-sample Mendelian randomization study. Eur J Neurol 30:1899–1906
Chen X, Yazdani S, Piehl F, Magnusson PKE, Fang F (2018) Polygenic link between blood lipids and amyotrophic lateral sclerosis. Neurobiol Aging 67:202.e201-202.e206
Michels S, Kurz D, Rosenbohm A et al (2023) Association of blood lipids with onset and prognosis of amyotrophic lateral sclerosis: results from the ALS Swabia registry. J Neurol 270:3082–3090
Freedman DM, Kuncl RW, Cahoon EK, Rivera DR, Pfeiffer RM (2018) Relationship of statins and other cholesterol-lowering medications and risk of amyotrophic lateral sclerosis in the US elderly. Amyotroph Lateral Scler Frontotemporal Degener 19:538–546
Sørensen HT, Riis AH, Lash TL, Pedersen L (2010) Statin use and risk of amyotrophic lateral sclerosis and other motor neuron disorders. Circ Cardiovasc Qual Outcomes 3:413–417
Jawaid A, Khan R, Polymenidou M, Schulz PE (2018) Disease-modifying effects of metabolic perturbations in ALS/FTLD. Mol Neurodegener 13:63
Ho WY, Chang JC, Lim K et al (2021) TDP-43 mediates SREBF2-regulated gene expression required for oligodendrocyte myelination. J Cell Biol 2021:220
Ingre C, Chen L, Zhan Y, Termorshuizen J, Yin L, Fang F (2020) Lipids, apolipoproteins, and prognosis of amyotrophic lateral sclerosis. Neurology 94:e1835–e1844
Mariosa D, Kamel F, Bellocco R, Ye W, Fang F (2015) Association between diabetes and amyotrophic lateral sclerosis in Sweden. Eur J Neurol 22:1436–1442
Kioumourtzoglou MA, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG (2015) Diabetes Mellitus, Obesity, and Diagnosis of Amyotrophic Lateral Sclerosis: A Population-Based Study. JAMA Neurol 72:905–911
D’Ovidio F, d’Errico A, Carnà P, Calvo A, Costa G, Chiò A (2018) The role of pre-morbid diabetes on developing amyotrophic lateral sclerosis. Eur J Neurol 25:164–170
Turner MR, Goldacre R, Ramagopalan S, Talbot K, Goldacre MJ (2013) Autoimmune disease preceding amyotrophic lateral sclerosis: an epidemiologic study. Neurology 81:1222–1225
Mariosa D, Kamel F, Bellocco R et al (2020) Antidiabetics, statins and the risk of amyotrophic lateral sclerosis. Eur J Neurol 27:1010–1016
Moglia C, Calvo A, Canosa A et al (2017) Influence of arterial hypertension, type 2 diabetes and cardiovascular risk factors on ALS outcome: a population-based study. Amyotroph Lateral Scler Frontotemporal Degener 18:590–597
Mandrioli J, Ferri L, Fasano A et al (2018) Cardiovascular diseases may play a negative role in the prognosis of amyotrophic lateral sclerosis. Eur J Neurol 25:861–868
Abdel Magid HS, Topol B, McGuire V, Hinman JA, Kasarskis EJ, Nelson LM (2022) Cardiovascular Diseases, Medications, and ALS: A Population-Based Case-Control Study. Neuroepidemiology 56:423–432
Xia K, Zhang L, Tang L, Huang T, Fan D (2022) Assessing the role of blood pressure in amyotrophic lateral sclerosis: a Mendelian randomization study. Orphanet J Rare Dis 17:56
Akamine S, Marutani N, Kanayama D et al (2020) Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep 10:20350
Nagel G, Kurz D, Peter RS et al (2023) Cystatin C based estimation of chronic kidney disease and amyotrophic lateral sclerosis in the ALS registry Swabia: associated risk and prognostic value. Sci Rep 13:19594
Zhan Y, Fang F (2019) Smoking and amyotrophic lateral sclerosis: A mendelian randomization study. Ann Neurol 85:482–484
de Jong SW, Huisman MHB, Sutedja NA et al (2012) Smoking, Alcohol Consumption, and the Risk of Amyotrophic Lateral Sclerosis: A Population-based Study. Am J Epidemiol 176:233–239
Liu G, Ou S, Cui H et al (2021) Head Injury and Amyotrophic Lateral Sclerosis: A Meta-Analysis. Neuroepidemiology 2021:1–9
Anderson EN, Gochenaur L, Singh A et al (2018) Traumatic injury induces stress granule formation and enhances motor dysfunctions in ALS/FTD models. Hum Mol Genet 27:1366–1381
Davis-Plourde KL, Mayeda ER, Lodi S et al (2022) Joint Models for Estimating Determinants of Cognitive Decline in the Presence of Survival Bias. Epidemiology 33:362–371
Acknowledgements
The authors thank all the participants of the ALSrisc Study for their valuable contributions.
Funding
Open access funding provided by Karolinska Institute. This study was supported by the European Research Council Starting Grant (MegaALS 802091), the US Center for Disease Control and Prevention (R01TS000324-01–00), the Swedish Research Council (2019–01088 and 2023–02428), Bjorklunds Fund, the Ulla-Carin Lindquist Foundation, Neuro Sweden, and Svenska Frimurar Orden.
Author information
Authors and Affiliations
Contributions
FF and CI contributed to the study conception and secured funding. Material preparation and data collection were performed by CI, CS, RP, KS, UK, CC, YH, EJ, and FF. Study design and data analysis were performed by CC. The first draft of the manuscript was written by CC, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
Caroline Ingre has consulted for Cytokinetics, Pfizer, BioArctic, Novartis, Tikomed, and Mitsubishi, and was a DMC member for Appelis Pharmaceutical. She is also a board member of Tobii Dynavox and a board member of the Stiching TRICALS Foundation; all outside the submitted work.
Ethical approval
The study was approved by the Swedish Ethical Review Authority (DNRs: 2014–1815-31–4, 2018–1605-31, and 2021–06397-02). Oral and written informed consent was collected from all study participants.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chourpiliadis, C., Seitz, C., Lovik, A. et al. Lifestyle and medical conditions in relation to ALS risk and progression—an introduction to the Swedish ALSrisc Study. J Neurol 271, 5447–5459 (2024). https://doi.org/10.1007/s00415-024-12496-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12496-w