Abstract
Background
Epidemiological data are sparse regarding the risk of stroke in patients with multiple sclerosis (MS).
Objective
To estimate the following: (1) the pooled prevalence of all-cause stroke, acute ischaemic stroke (AIS) and intracerebral haemorrhage (ICH) in MS patients; (2) the relative risk for all-cause stroke, AIS and ICH in MS patients compared to the general population; (3) associations between patient characteristics and the risk for AIS and ICH in MS patients.
Methods
Systematic review and meta-analysis of registry-based and cohort studies.
Results
Thirteen observational studies comprising 146,381 MS patients were included. The pooled prevalence of all-cause stroke was 2.7% (95% confidence interval [CI] 1.3–4.6%), with the relative risk of all-cause stroke being higher in MS patients compared to the general population (RR: 2.55; 95% CI 1.97–3.29). Subgroup analyses per stroke subtype revealed a pooled AIS prevalence of 2.1% (95% CI 0.8–4.1%) and a pooled ICH prevalence of 0.6% (95% CI 0.2–1.2%). Compared to the general population, patients with MS were found to harbour an increased risk for AIS (RR: 2.79; 95% CI 2.27–3.41) and ICH (RR: 2.31; 95% CI 1.04–5.11), respectively. The pooled prevalence of cardiovascular risk factors in MS patients was 11.5% (95% CI 2.9–24.7%) for dyslipidaemia, 18.2% (95% CI 5.9–35.3%) for hypertension and 5.4% (95% CI 2.1–10.2%) for diabetes. In meta-regression, age was negatively associated with AIS risk (β = – .03, p = 0.04), with a 1-year increase in age resulting in a significant 3% (95%CI 0–5) attenuation of the risk of AIS.
Conclusion
The findings of the present meta-analysis indicate that MS is associated with an increased risk for ischaemic and haemorrhagic stroke. Future well-designed epidemiological studies are warranted to corroborate the robustness of the present findings in the MS population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) comprises a chronic inflammatory demyelinating disease of the human central nervous system, which (despite the recent tremendous therapeutic advances) still ranks as a leading cause of neurological disability among young and middle-aged adults worldwide [1]. With growing evidence, it has become apparent that the epidemiological landscape of MS continues to evolve, with population-specific genetic and environmental factors propelling changes in MS epidemiological metrics [2, 3]. Since the global disease burden of MS is rising, epidemiological studies disclose an exponential increase in the prevalence and incidence of MS across several geographic regions. These findings have largely been attributed to (i) improved survival of patients with MS, (ii) earlier MS diagnosis and (iii) shift in gene–environment interplays that exacerbate MS [2, 3].
On the other hand, disability accrual in MS has been increasingly acknowledged as not solely mediated by relapse-associated worsening, but also by disease progression independent of relapse activity [4]. With respect to the latter, it remains to date equivocal whether the accumulation of disability is strictly mediated by disease-specific immunological processes. An alternative hypothesis postulates that sustained blood–brain barrier (BBB) disruption, vascular changes and hypoxic cascades may partly contribute to relapse-independent neurodegeneration [5, 6]. In fact, epidemiological studies indicate that the patients with MS harbour an increased risk for vascular comorbidities and cerebrovascular diseases that may account for enhanced neurodegeneration and disability progression [7]. Beyond the epidemiological link, large-scale genetic and basic research studies have also recently suggested that neurovascular dysfunction may comprise a pathophysiologically intrinsic, albeit underrecognized, facet of MS [8]. The extent to which MS may correlate with cerebrovascular disease remains to be established.
In view of the former considerations, the aim of the present systematic review and meta-analysis was threefold. First, we sought to estimate the prevalence of all-cause stroke, acute ischaemic stroke (AIS) and intracerebral haemorrhage (ICH) in MS patients. Second, we attempted to examine the relative risk for all-cause stroke, AIS and ICH in patients with MS compared to the general population. Third, we aimed to investigate vascular comorbidities and patient characteristics associated with the AIS and ICH risk in MS patients.
Methods
Standard protocol approvals and registrations
The protocol for the present systematic review and meta-analysis has been pre-registered to the Open Search Foundation (OSF) (Registration: osf.io/7djhf). The updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9] have been employed for reporting, while reporting also adheres to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) proposal [10]. Ethical board approval and individual written informed consent were not required for the present study as per the study design (systematic review and meta-analysis).
Data sources, searches and study selection
A systematic literature search was independently performed by two reviewers (MIS, VG) to identify eligible studies that reported on AIS or ICH in MS patients. MEDLINE, Cochrane Library and SCOPUS databases were searched by applying search strings comprising the following search terms: “multiple sclerosis”, “stroke”, “intracranial haemorrhage”, “small vessel disease”, “vascular risk”, “vascular comorbidities” and “cardiovascular risk”. The full search algorithms that were used in MEDLINE, Cochrane Library and SCOPUS databases have been provided in the Supplement. The search spanned from each electronic database’s inception to 21 January 2024. To ascertain the comprehensiveness of the bibliography, reference lists of published articles fulfilling our inclusion criteria were searched manually.
Clinical trials, population-based studies or registries, along with observational cohort studies that reported on AIS or ICH in MS patients, were eligible for inclusion. Patients diagnosed with MS were considered eligible for inclusion, provided that the patient’s diagnosis was either with relapsing–remitting MS (RRMS), primary progressive MS (PPMS) or secondary progressive MS (SPMS). Per study protocol, studies were excluded if: (1) MS/AIS/ICH diagnoses were uncertain according to our pre-defined inclusion criteria; (2) reported outcomes were not aligned with our inclusion criteria; (3) and they were case reports, case series, narrative and systematic reviews, commentaries, pre-prints or non-peer reviewed studies and conference abstracts. In case that studies had overlapping data, we retained the study with the largest dataset. All retrieved studies were assessed by two reviewers (MIS, VG) independently, and any disagreements between reviewers were resolved after discussion with a third tie-breaking evaluator (GT).
Quality control, bias assessment and data extraction
For relevant domains of each included study, the risk of bias was assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [11]. Two independent reviewers (MIS, VG) performed quality control and bias assessment, and consensus after discussion with the corresponding author (SG) was reached in case of disagreement. For further analyses, data including author names, date of publication, study design, country, event type (i.e. all-cause stroke, AIS or ICH) and patient characteristics were extracted from individual studies in structured reports.
Outcomes
We performed an aggregate data meta-analysis by including identified population-based studies or registries, and observational cohort studies.
The pre-defined primary outcome measures of the present meta-analysis were twofold: (i) the pooled prevalence of all-cause stroke, AIS and ICH in MS patients and (ii) the relative risk for all-cause stroke, AIS and ICH in MS patients compared to the general population. For relative risk assessment, only studies that provided estimates in both MS patients and the general population (i.e. controls) were considered. Secondary outcomes of interest comprised the prevalence of vascular comorbidities in patients with MS. Additionally, associations between demographic characteristics and MS-related characteristics and AIS or ICH diagnosis were assessed in the MS population. Sensitivity analyses were also performed after the exclusion of low-quality studies and after the exclusion of studies with the duration shorter than 10 years.
Statistical analysis
For each dichotomous outcome of interest, the pooled prevalence with 95% confidence intervals (95% CI) was calculated for the aggregate meta-analysis, after the implementation of the Freeman–Tukey variance-stabilizing double arcsine transformation [12,13,14]. The random-effects model of meta-analysis (DerSimonian and Laird) was utilized for estimation of the pooled estimates [15]. Pairwise comparisons were conducted between MS patients and the general population and were reported using risk ratios (RRs) and corresponding 95% confidence intervals (95% CI). We used the Q test to assess subgroup differences [16]. Accordingly, the I2 and Cochran Q statistics were employed for heterogeneity assessment. With respect to the qualitative heterogeneity interpretation, I2 values > 50% and values > 75% were regarded to represent either substantial or considerable heterogeneity, respectively. The significance level was set at 0.1 for the Q statistic. Graphical assessment of publication bias was performed across individual studies when more than four studies were included for each primary outcome-of-interest analysis, we used a funnel and radial plot inspection and the Egger’s linear regression test accordingly [17], while the equivalent z test with a two-tailed p value < 0.05 was considered statistically significant for each pooled estimate. All statistical analyses and figure production were carried out using RStudio for Windows [R studio/R Meta package].
Results
Literature search and included studies
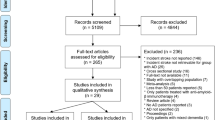
The systematic database search yielded 14,112 records from MEDLINE, SCOPUS and Cochrane Library databases. After the exclusion of duplicates and articles that were out of scope, 1,261 records were considered eligible for inclusion and were assessed in full. After reading the full-text articles, 1,248 were further excluded (Supplement). Finally, we identified 13 eligible studies [18,19,20,21,22,23,24,25,26,27,28,29,30] for inclusion comprising a total of 146,381 MS patients. All retrieved studies were observational, and Table 1 summarizes their main characteristics, including country of origin, study type, population size and reported outcomes. In Fig. 1, the PRISMA flow chart of the meta-analysis is presented.
Quality control of included studies
The risk of bias of studies included in the present meta-analysis was assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [11] and is presented in Supplementary Fig. 1. The majority of studies presented significant biases due to confounding (i.e. by not reporting key confounding variables such as MS subtypes, disability status or stroke characteristics), as well as biases in the classification of primary outcomes (i.e. diagnostic criteria not reported) (Table 2).
Quantitative analyses
Primary outcome
A total of 146,381 MS patients were included in the meta-analysis. MS diagnosis was ascertained by the use of International Classification of Diseases (ICD) codes in all included studies, with the exception of the study by Tadic et al. [29] which reported that only patients with “definitive MS” were included. The pooled prevalence of all-cause stroke among MS patients was 2.7% (95% CI 1.3–4.6%;13 studies; I2 = 100%, p for Cochran Q = 0; Fig. 2) [18,19,20,21,22,23,24,25,26,27,28,29,30], with the relative risk of all-cause stroke being significantly higher in MS patients compared to the general population (RR: 2.55; 95% CI 1.97–3.29; 11 studies; I2 = 96%; p for Cochran Q < 0.01)[18,19,20,21,22,23,24,25,26,27,28,29,30] (Fig. 3). When the data were stratified according to stroke subtype, the pooled prevalence of AIS in MS patients was 2.1% (95% CI 0.8–4.1%; eight studies; I2 = 100%, p for Cochran Q = 0; Fig. 4) [18, 19, 21, 23, 24, 26, 28, 30]; the relative risk of AIS in MS patients compared to the general population was 2.79 (95% CI 2.28–3.42; seven studies; I2 = 84%; p for Cochran Q < 0.01; Fig. 5) [18, 19, 21, 23, 24, 26, 28, 30]. With respect to ICH, the pooled ICH prevalence in MS patients was 0.6% (95% CI 0.2–1.2%; four studies; I2 = 98%, p for Cochran Q < 0.01; Fig. 6) [18, 21, 24, 30]; the relative risk for ICH in patients with MS compared to the general population was 2.31 (95% CI 1.05–5.12; three studies; I2 = 94%; p for Cochran Q < 0.01; Fig. 7) [18, 21, 24, 30].
Secondary outcomes
With respect to cardiovascular risk factors, the pooled prevalence of hypertension, diabetes and dyslipidaemia were assessed in MS patients. The pooled prevalence of hypertension was 18.2% (95% CI 5.9–35.3%; seven studies; I2 = 100%; p for Cochran Q = 0, Supplementary Fig. 2) in MS patients [20, 22,23,24,25, 29, 30]. The pooled prevalence of diabetes and dyslipidaemia was 5.4% (95% CI 2.1–10.2%; six studies; I2 = 99%; p for Cochran Q < 0.01; Supplementary Fig. 3) [19, 23, 24, 28,29,30] and 11.5% (95% CI 2.9–24.7%; six studies; I2 = 100%; p for Cochran Q = 0; Supplementary Fig. 4) [20, 23, 24, 28,29,30], respectively.
With respect to the association between characteristics of MS patients and the risk of stroke, a subgroup analysis was performed including only studies that reported the mean age of MS patients. In particular, meta-regression was possible for all-cause stroke and AIS patients, but not for individuals with ICH due to data unavailability. Meta-regression analysis revealed that age is not a significant predictor neither of all-cause stroke (p = 0.96), nor AIS (p = 0.57). No significant association was disclosed between age and the risk of all-cause stroke (p = 0.66). However, there was a statistically significant negative association between age and the risk of AIS (β = – 0.03; 95% CI – 0.06–0, p = 0.04), with a 1-year increase in age resulting in a significant 3% (95% CI 0–5) attenuation of AIS risk [18, 23, 24, 28] (Supplementary Fig. 5).
Sensitivity analysis
Sensitivity analyses were subsequently performed to assess the prevalence and relative risk of all-cause stoke after the exclusion of low-quality studies [20, 29]. The pooled prevalence of all-cause stroke was 2.5% (95% CI 1.1–4.6%; 11 studies; I2 = 100%, p for Cochran Q = 0; Supplementary Fig. 6), while the relative risk for all-cause stroke between patients with MS and the general population was 2.41 (95% CI 1.91–3.04; nine studies; I2 = 96%, p for Cochran Q < 0.01; Supplementary Fig. 7). Furthermore, an additional analysis after the exclusion of studies with the duration shorter than 10 years [20, 22, 24, 28, 30] revealed a 2.1% pooled prevalence for all-cause stroke (95% CI 0.9–3.9%; seven studies; I2 = 100%; p for Cochran Q = 0; Supplementary Fig. 8) and a relative risk for all-cause stroke of 1.65 (95% CI 0.98–2.76; five studies; I2 = 98%, p for Cochran Q < 0.01; Supplementary Fig. 9). Sensitivity analyses stratified by stroke subtype could not be performed due to data unavailability.
Publication bias
Funnel plots were employed to evaluate publication bias for the primary outcome of interest. Regarding all-cause stroke prevalence, moderate to high funnel plot asymmetry was uncovered (Supplementary Fig. 10) with a statistically significant Egger’s test (p < 0.01). Regarding the risk ratio of all-cause stroke in MS patients compared to the general population, funnel plot inspection revealed moderate asymmetry with a non-significant Egger’s test (p = 0.17) (Supplementary Fig. 11).
Discussion
In the present systematic review and meta-analysis, the pooled prevalence of all-cause stroke among patients with MS was estimated at 2.7 cases per 100 patients (95%CI 1.3–4.6%), with the relative risk of all-cause stroke being more than twofold higher in patients with MS compared to the general population (RR:2.55; 95%CI 1.97–3.29). Additionally, an increased predisposition of patients with MS to ischaemic rather than haemorrhagic stroke was disclosed, as indicated by the higher cumulative prevalence of AIS of 2.1% (95%CI 0.8–4.1%) as opposed to the cumulative ICH prevalence of 0.6% (95%CI 0.2–1.2%). Accordingly, comparisons between patients with MS and the general population revealed that MS patients harbour an increased risk for AIS and ICH, respectively.
These findings are in accordance with the results of prior population-based cohorts and registries that ascertain an increased risk of MS patients for cerebrovascular and cardiovascular diseases [12, 31]. In particular, cardiovascular risk factors were documented with varying prevalence among patients with MS, with crude prevalence estimates for hypertension of 18.2% (95% CI 5.9–35.3%), diabetes: 5.4% (95% CI 2.1–10.2%) and dyslipidaemia: 11.5% (95% CI 2.9–24.7). Notably, vascular comorbidities in MS have been previously linked to (i) the sedentary lifestyle and immobility of MS patients [32, 33], (ii) the excess risk for psychiatric comorbidities [34], (iii) MS-related cardiac autonomic dysfunction[35] and (iv) shared genetic variants between MS and cerebrovascular diseases as revealed by large-scale genome-wide association studies [36, 37]. Notwithstanding the evidence of a heightened risk for vascular comorbidities, however, recent studies suggest that MS may comprise an independent risk factor for cerebrovascular disease (i.e. with the cerebrovascular risk in MS not fully accounted for by traditional vascular risk factors) [38, 39].
From a pathophysiological perspective, several MS-specific mechanisms have been implicated in incident cerebrovascular disease in MS patients. First, chronic inflammation has been linked to endothelial dysfunction and distinct arteriolar changes (i.e. arteriolosclerosis, periarteriolar space dilatation and hemosiderin deposition), which in turn precipitate micro-ischaemia and micro-haemorrhages within the cerebral white matter [6, 40]. Second, pathological studies comparing patients with MS to age-matched controls have disclosed a significant correlation between cerebral small vessel disease—but not large artery atherosclerosis—and MS [6]. Third, the use of certain disease-modifying therapies (DMTs) has been associated with cardiovascular complications, including cardiotoxicity, haemodynamic impairment, hypertensive derailment and increased risk for ICH (e.g. alemtuzumab) [41,42,43,44]. With respect to the latter, although we cannot exclude that surveillance and reporting biases may partly account for an overestimation of the crude ICH prevalence in patients with MS, it is striking that the recorded proportion of ischaemic over haemorrhagic stroke in patients with MS was aligned with to the global, epidemiological, age-standardized 3.5:1 ratio of ischaemic vs. haemorrhagic stroke [45]. To the best of our knowledge, the present meta-analysis is the first to provide evidence of an increased predisposition of patients with MS to ICH. With the established venocentric progression of MS lesions in mind, chronic cerebrospinal venous insufficiency and hampered cerebrospinal venous drainage may comprise additional pathways that independently confer an enhanced risk for cerebral microbleeds and ICH in patients with MS [46, 47].
In addition, we found a significant negative association between age and the risk of AIS in patients with MS, with a 1-year increase in age resulting in a significant 3% attenuation of the risk for AIS. These results are aligned with evidence from previous studies that indicate a time-dependent decrease in the risk of stroke in MS patients, but also in patients with other immune-mediated diseases (including ankylosing spondylitis, polymyalgia rheumatica, rheumatoid arthritis, systemic lupus erythematosus, Wegener’s granulomatosis, Crohn’s disease, ulcerative colitis, immune thrombocytopenic purpura, polymyositis/dermatomyositis and Sjögren’s syndrome) [21]. Several mechanisms may account for the previous findings, including (i) haemostatic imbalance (i.e. procoagulant upregulation, anticoagulant downregulation and fibrinolysis suppression) in the setting of untreated immune-mediated disease [48], (ii) pro-coagulatory effects of corticosteroids [49], and (iii) decreasing inflammatory activity and hence cardiovascular risk following DMT initiation [50]. Although data on DMTs were not available for meta-regression, it would be compelling for future studies to assess the potential association of DMTs with the risk of stroke in MS patients.
Taken together, the results of the present meta-analysis expand and strengthen the findings of previous research [51], by incorporating data from recently published studies [26,27,28,29,30], while providing prevalence estimates per stroke subtype and cardiovascular comorbidity in the MS population. Nonetheless, certain limitations should be acknowledged for an accurate interpretation of our findings. First, there was significant heterogeneity among included studies, both in terms of methodology and population characteristics, which may have confounded the pooled prevalence estimates. Nevertheless, sensitivity analyses after the exclusion of low-quality studies and studies with the duration shorter than 10 years did not affect the pooled estimates. Further epidemiological research is warranted to evaluate the generalizability of the present results. Second, all included studies were observational and may have suffered from selection or reporting biases, a fact that hinders robust inferences from noted associations. Third, only a limited number of studies provided data for meta-regression; importantly, disability parameters (i.e. Expanded Disability Status Scale—EDSS), stroke aetiology [52, 53], DMTs and MS subtypes were not systematically documented in included studies. Thus, further subgroup or sensitivity analyses could not be performed. Consequently, larger well-characterized cohorts and registries (i.e. providing individual patient or stratified data including EDSS, stroke aetiology, MS subtype and DMTs) are urgently required to delineate stroke characteristics in MS patients with the aim to unravel causal associations between MS and stroke. Fourth, residual confounding due to publication bias cannot be excluded; thus, replication of the present results is warranted in the context of large, multicentre observational and epidemiological studies.
In conclusion, the findings of the present meta-analysis indicate a positive association between MS and risk of all-cause stroke, AIS and ICH. Given the aforementioned methodological limitations, including the high heterogeneity in reported outcomes, potential presence of publication bias and overall moderate quality of studies included in the present meta-analysis, the future well-designed epidemiological research is required to corroborate our findings. Beyond traditional cardiovascular risk factors that confer a heightened risk of stroke in MS patients, the evidence of a “paradoxical” attenuation of the risk of stroke with increasing patient age may be aligned with the hypothesis of disease activity comprising an independent risk factor for cerebrovascular disease in MS. While further research is required to elaborate the pathophysiological associations behind this correlation, clinicians should recognize the elevated stroke risk and prioritize targeted stroke prevention strategies in the MS patient population to reduce stroke burden and improve patient outcomes.
Data availability
All data analysed in the present study have been included in the present article and its supplementary material.
References
Strober LB, Christodoulou C, Benedict RH, Westervelt HJ, Melville P, Scherl WF, Weinstock-Guttman B, Rizvi S, Goodman AD, Krupp LB (2012) Unemployment in multiple sclerosis: the contribution of personality and disease. Mult Scler 18:647–653
Koch-Henriksen N, Sørensen PS (2010) The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 9:520–532
Leray E, Moreau T, Fromont A, Edan G (2016) Epidemiology of multiple sclerosis. Rev Neurol (Paris) 172:3–13
Lublin FD, Häring DA, Ganjgahi H, Ocampo A, Hatami F, Čuklina J, Aarden P, Dahlke F, Arnold DL, Wiendl H, Chitnis T, Nichols TE, Kieseier BC, Bermel RA (2022) How patients with multiple sclerosis acquire disability. Brain 145:3147–3161
Spencer JI, Bell JS, DeLuca GC (2018) Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood-brain barrier. J Neurol Neurosurg Psychiatry 89:42–52
Geraldes R, Esiri MM, Perera R, Yee SA, Jenkins D, Palace J, DeLuca GC (2020) Vascular disease and multiple sclerosis: a post-mortem study exploring their relationships. Brain 143:2998–3012
Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T (2010) Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 74:1041–1047
Kaplan TB, Berkowitz AL, Samuels MA (2015) Cardiovascular dysfunction in multiple sclerosis. Neurologist 20:108–114
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Moher D (2021) Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 134:103–112
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Giannopapas V, Palaiodimou L, Kitsos D, Papagiannopoulou G, Stavrogianni K, Chasiotis A, Kosmidou M, Tzartos JS, Paraskevas GP, Bakalidou D, Tsivgoulis G, Giannopoulos S (2023) The prevalence of Diabetes Mellitus Type II (DMII) in the Multiple sclerosis population: a systematic review and meta-analysis. J Clin Med 12:4948
Stefanou MI, Palaiodimou L, Katsanos AH, Milionis H, Kosmidou M, Lambadiari V, Halvatsiotis P, Ferentinos P, Andreadou E, Marinos G, Theodorou A, Tzartos JS, Voumvourakis K, Tsivgoulis G, Giannopoulos S (2022) The effects of HMG-CoA reductase inhibitors on disease activity in multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord 58:103395
Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Annals Math Stat 607–611
Tsivgoulis G, Katsanos AH, Köhrmann M, Caso V, Perren F, Palaiodimou L, Deftereos S, Giannopoulos S, Ellul J, Krogias C (2019) Duration of implantable cardiac monitoring and detection of atrial fibrillation in ischemic stroke patients: a systematic review and meta-analysis. J Stroke 21:302
Borenstein M, Higgins JP (2013) Meta-analysis and subgroups. Prev Sci 14:134–143
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Allen NB, Lichtman JH, Cohen HW, Fang J, Brass LM, Alderman MH (2008) Vascular disease among hospitalized multiple sclerosis patients. Neuroepidemiology 30:234–238
Christiansen CF, Christensen S, Farkas DK, Miret M, Sørensen HT, Pedersen L (2010) Risk of arterial cardiovascular diseases in patients with multiple sclerosis: a population-based cohort study. Neuroepidemiology 35:267–274
Lavela SL, Prohaska TR, Furner S, Weaver FM (2012) Chronic diseases in male veterans with multiple sclerosis. Prev Chronic Dis 9:E55
Zöller B, Li X, Sundquist J, Sundquist K (2012) Risk of subsequent ischemic and hemorrhagic stroke in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. BMC Neurol 12:41
Jadidi E, Mohammadi M, Moradi T (2013) High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult Scler 19:1336–1340
Tseng CH, Huang WS, Lin CL, Chang YJ (2015) Increased risk of ischaemic stroke among patients with multiple sclerosis. Eur J Neurol 22:500–506
Capkun G, Dahlke F, Lahoz R, Nordstrom B, Tilson HH, Cutter G, Bischof D, Moore A, Simeone J, Fraeman K, Bancken F, Geissbühler Y, Wagner M, Cohan S (2015) Mortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: an observational study using the US Department of Defense administrative claims database. Mult Scler Relat Disord 4:546–554
Thormann A, Magyari M, Koch-Henriksen N, Laursen B, Sørensen PS (2016) Vascular comorbidities in multiple sclerosis: a nationwide study from Denmark. J Neurol 263:2484–2493
Persson R, Lee S, Yood MU, Wagner M, Minton N, Niemcryk S, Lindholm A, Evans A, Jick S (2020) Incident cardiovascular disease in patients diagnosed with multiple sclerosis: a multi-database study. Mult Scler Relat Disord 37:101423
Zulfiqar M, Qeadan F, Ikram A, Farooqui M, Richardson SP, Calder CS, Quadri SA, Mathur P, Ford C, Liera E, Snow H (2019) Intracerebral hemorrhage in multiple sclerosis: a retrospective cohort study. J Stroke Cerebrovasc Dis 28:267–275
Castelo-Branco A, Chiesa F, Bengtsson CE, Lee S, Minton NN, Niemcryk S, Lindholm A, Rosenlund M, Piehl F, Montgomery S (2020) Non-infectious comorbidity in patients with multiple sclerosis: a national cohort study in Sweden. Mult Scler J Exp Transl Clin 6:2055217320947761
Tadić D, Grgić S, Dominović-Kovačević A, Nazalević-Bursać M, Mavija S, Đajić V (2022) Vascular comorbidities in patients with multiple sclerosis and their impact on physical disability. Med Glas (Zenica) 19:166–172
Cho EB, Yeo Y, Jung JH, Jeong SM, Han KD, Shin DW, Min JH (2022) Risk of stroke in multiple sclerosis and neuromyelitis optic spectrum disorder: a Nationwide cohort study in South Korea. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2022-329628
Christiansen CF (2012) Risk of vascular disease in patients with multiple sclerosis: a review. Neurol Res 34:746–753
Veldhuijzen van Zanten JJ, Pilutti LA, Duda JL, Motl RW (2016) Sedentary behaviour in people with multiple sclerosis: Is it time to stand up against MS? Mult Scler 22:1250–1256
Roshanisefat H, Bahmanyar S, Hillert J, Olsson T, Montgomery S (2014) Multiple sclerosis clinical course and cardiovascular disease risk - Swedish cohort study. Eur J Neurol 21:e1353–e1388
Thormann A, Sørensen PS, Koch-Henriksen N, Laursen B, Magyari M (2017) Comorbidity in multiple sclerosis is associated with diagnostic delays and increased mortality. Neurology 89:1668–1675
Racosta JM, Sposato LA, Morrow SA, Cipriano L, Kimpinski K, Kremenchutzky M (2015) Cardiovascular autonomic dysfunction in multiple sclerosis: a meta-analysis. Mult Scler Relat Disord 4:104–111
Tian Z, Song Y, Yao Y, Guo J, Gong Z, Wang Z (2020) Genetic etiology shared by multiple sclerosis and ischemic stroke. Front Genet 11:646
Zeng R, Jiang R, Huang W, Wang J, Zhang L, Ma Y, Wu Y, Meng M, Lan H, Lian Q, Leung FW, Sha W, Chen H (2023) Dissecting shared genetic architecture between obesity and multiple sclerosis. EBioMedicine 93:104647
Palladino R, Chataway J, Majeed A, Marrie RA (2021) Interface of multiple sclerosis, depression, vascular disease, and mortality: a population-based matched cohort study. Neurology 97:e1322–e1333
Palladino R, Marrie RA, Majeed A, Chataway J (2020) Evaluating the risk of macrovascular events and mortality among people with multiple sclerosis in England. JAMA Neurol 77:820–828
Senzaki K, Okada Y, Ochi H, Ochi M, Takei SI, Miura S, Igase M, Ohyagi Y (2021) Vascular endothelial dysfunction associated with severity in multiple sclerosis. Mult Scler Relat Disord 54:103135
Al-Yafeai Z, Carvajal-González A, Abduljabar H, Arvas M, Patel S, Patel N (2022) Novel multiple sclerosis agents-associated cardiotoxicity: a real-world pharmacovigilance study. Int J Cardiol 362:153–157
Najafian J, Nasri A, Etemadifar M, Salehzadeh F (2019) Late Cardiotoxicity in MS patients treated with mitoxantrone. Int J Prev Med 10:211
Sánchez-Soblechero A, Cuello JP, Martínez Ginés ML, Lozano Ros A, Romero Delgado F, De Andrés C, Goicochea Briceño H, García Domínguez JM (2022) Recurrent intracranial hemorrhage in a patient with relapsing multiple sclerosis under interferon-β therapy. Neurologia (Engl Ed) 37:77–79
Azevedo CJ, Kutz C, Dix A, Boster A, Sanossian N, Kaplan J (2019) Intracerebral haemorrhage during alemtuzumab administration. Lancet Neurol 18:329–331
Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, Abbasifard M, Abbasi-Kangevari M, Abd-Allah F, Abedi V (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20:795–820
Sati P, Oh J, Constable RT, Evangelou N, Guttmann CR, Henry RG, Klawiter EC, Mainero C, Massacesi L, McFarland H, Nelson F, Ontaneda D, Rauscher A, Rooney WD, Samaraweera AP, Shinohara RT, Sobel RA, Solomon AJ, Treaba CA, Wuerfel J, Zivadinov R, Sicotte NL, Pelletier D, Reich DS (2016) The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 12:714–722
Zhang R, Li Q, Zhou Y, Yan S, Zhang M, Lou M (2019) The relationship between deep medullary veins score and the severity and distribution of intracranial microbleeds. Neuroimage Clin 23:101830
Xu J, Lupu F, Esmon CT (2010) Inflammation, innate immunity and blood coagulation. Hamostaseologie 30(5–6):8–9
Jilma B, Cvitko T, Winter-Fabry A, Petroczi K, Quehenberger P, Blann AD (2005) High dose dexamethasone increases circulating P-selectin and von Willebrand factor levels in healthy men. Thromb Haemost 94:797–801
Sternberg Z, Leung C, Sternberg D, Yu J, Hojnacki D (2014) Disease modifying therapies modulate cardiovascular risk factors in patients with multiple sclerosis. Cardiovasc Ther 32:33–39
Hong Y, Tang HR, Ma M, Chen N, Xie X, He L (2019) Multiple sclerosis and stroke: a systematic review and meta-analysis. BMC Neurol 19:139
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Meretoja A, Strbian D, Putaala J, Curtze S, Haapaniemi E, Mustanoja S, Sairanen T, Satopää J, Silvennoinen H, Niemelä M, Kaste M, Tatlisumak T (2012) SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke 43:2592–2597
Acknowledgements
None.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
MIS, VG, SG and GT contributed to conception and study design. MIS, VG, SG and GT contributed to acquisition and analysis of data. MIS, VG, SG and GT contributed to drafting a significant portion of the manuscript or figures. DK, MC, AT, MK, PV, CB, EA and JST contributed with critical comments during manuscript revision.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Stefanou—nothing to report. Dr. Giannopapas—nothing to report. Dr. Kitsos—nothing to report. Dr. Chondrogianni—nothing to report. Dr. Theodorou—nothing to report. Dr. Kosmidou—nothing to report. Dr. Vlotinou—nothing to report. Dr. Bakirtzis—nothing to report. Dr. Andreadou—nothing to report. Dr. Tzartos—nothing to report. Dr. Giannopoulos—nothing to report. Dr. Tsivgoulis—nothing to report.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stefanou, MI., Giannopapas, V., Kitsos, D.K. et al. Prevalence and epidemiology of stroke in patients with multiple sclerosis: a systematic review and meta-analysis. J Neurol 271, 4075–4085 (2024). https://doi.org/10.1007/s00415-024-12331-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12331-2