Abstract
Objective
Alteplase is the current standard of care for acute ischemic stroke. Tenecteplase is a newer fibrinolytic agent with preferable administration and lower costs; however, its comparative effectiveness to alteplase remains uncertain. We set out to perform a systematic review and meta-analysis to establish the benefits and harms of tenecteplase versus alteplase for acute ischemic stroke.
Methods
We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov from inception to April 2023 for randomized and non-randomized studies that compared tenecteplase versus alteplase for acute ischemic stroke. Paired reviewers independently assessed risk of bias and extracted data. We performed both conventional meta-analyses and Bayesian network meta-analyses (NMA) with random-effects models and used the GRADE approach to evaluate the certainty of evidence. Our primary efficacy outcome was excellent functional outcome at 3 months, defined as a score of 0–1 on the modified Rankin Scale. Our primary safety outcomes were symptomatic intracranial hemorrhage and all-cause mortality.
Results
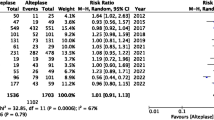
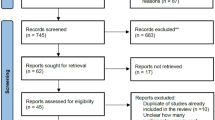
Thirty-six studies were eligible for review, including 12 randomized (n = 5533) and 24 non-randomized studies (n = 44,956). Moderate certainty evidence showed that there was no difference between tenecteplase and alteplase in increasing the proportion of patients achieving excellent functional outcome at 3 months (odds ratio [OR], 1.10; 95% CI 0.98–1.23; risk difference [RD] 2.4%, 95% CI − 0.5 to 5.2), while moderate certainty evidence from NMA suggested that 0.25 mg/kg tenecteplase significantly improved excellent functional outcome at 3 months (OR, 1.16; 95% credible interval 1.02–1.32). Moderate certainty evidence showed that, compared to alteplase, tenecteplase may make little to no difference in the prevalence of symptomatic intracranial hemorrhage (OR, 1.12; 95% CI 0.79–1.59; RD 0.3%, 95% CI − 0.5 to 1.4), and probably reduces all-cause mortality (adjusted odds ratio [aOR], 0.44; 95% CI 0.30–0.64; RD − 4.6%; 95% CI − 5.8 to − 2.9).
Conclusions
Moderate certainty evidence suggested that there was little to no difference between tenecteplase and alteplase in increasing the proportion of patients achieving excellent functional outcome at 3 months and the risk of symptomatic intracranial hemorrhage, while compared to alteplase, tenecteplase probably reduce all-cause mortality. Administration of 0.25 mg/kg tenecteplase after acute ischemic stroke is suggestive of increasing the proportion of patients that achieve excellent functional outcome at 3 months.



Similar content being viewed by others
Data availability
No additional data available.
References
Phipps MS, Cronin CA (2020) Management of acute ischemic stroke. BMJ 368:l6983
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS (2023) Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation 147(8):e93–e621
(2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 20(10):795–820
Saver JL, Adeoye O (2021) Intravenous thrombolysis before endovascular thrombectomy for acute ischemic stroke. JAMA 325(3):229–231
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50(12):e344–e418
Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, de la Ossa NP, Strbian D, Tsivgoulis G, Turc G (2021) European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 6(1):I–lxii
Bivard A, Huang X, McElduff P, Levi CR, Campbell BC, Cheripelli BK, Kalladka D, Moreton FC, Ford I, Bladin CF, Davis SM, Donnan GA, Muir KW, Parsons MW (2017) Impact of computed tomography perfusion imaging on the response to tenecteplase in ischemic stroke: analysis of 2 randomized controlled trials. Circulation 135(5):440–448
Schwamm LH (2015) Breaking up is hard to do: tenecteplase in acute stroke. Lancet Neurol 14:343–345
Tanswell P, Modi N, Combs D, Danays T (2002) Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 41(15):1229–1245
Warach SJ, Dula AN, Milling TJ Jr (2020) Tenecteplase thrombolysis for acute ischemic stroke. Stroke 51(11):3440–3451
Nepal G, Kharel G, Ahamad ST, Basnet B (2018) Tenecteplase versus alteplase for the management of acute ischemic stroke in a low-income country-nepal: cost, efficacy, and safety. Cureus 10(2):e2178
Gao L, Moodie M, Mitchell PJ, Churilov L, Kleinig TJ, Yassi N, Yan B, Parsons MW, Donnan GA, Davis SM, Campbell BCV (2020) Cost-effectiveness of tenecteplase before thrombectomy for ischemic stroke. Stroke 51(12):3681–3689
Turc G, Hadziahmetovic M, Walter S, Churilov L, Larsen K, Grotta JC, Yamal JM, Bowry R, Katsanos AH, Zhao H, Donnan G, Davis SM, Hussain MS, Uchino K, Helwig SA, Johns H, Weber JE, Nolte CH, Kunz A, Steiner T, Sacco S, Ebinger M, Tsivgoulis G, Fassbender K, Audebert HJ (2022) Comparison of mobile stroke unit with usual care for acute ischemic stroke management: a systematic review and meta-analysis. JAMA Neurol 79(3):281–290
Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Dewey HM, Thijs V, Scroop R, Simpson M, Brooks M, Asadi H, Wu TY, Shah DG, Wijeratne T, Ang T, Miteff F, Levi CR, Rodrigues E, Zhao H, Salvaris P, Garcia-Esperon C, Bailey P, Rice H, de Villiers L, Brown H, Redmond K, Leggett D, Fink JN, Collecutt W, Wong AA, Muller C, Coulthard A, Mitchell K, Clouston J, Mahady K, Field D, Ma H, Phan TG, Chong W, Chandra RV, Slater LA, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Bladin CF, Sharma G, Desmond PM, Parsons MW, Donnan GA, Davis SM (2018) Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 378(17):1573–1582
Bivard A, Zhao H, Churilov L, Campbell BCV, Coote S, Yassi N, Yan B, Valente M, Sharobeam A, Balabanski AH, Dos Santos A, Ng JL, Yogendrakumar V, Ng F, Langenberg F, Easton D, Warwick A, Mackey E, MacDonald A, Sharma G, Stephenson M, Smith K, Anderson D, Choi P, Thijs V, Ma H, Cloud GC, Wijeratne T, Olenko L, Italiano D, Davis SM, Donnan GA, Parsons MW (2022) Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): a phase 2, randomised, open-label trial. Lancet Neurol 21(6):520–527
Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, O’Brien B, Bladin C, McElduff P, Allen C, Bateman G, Donnan G, Davis S, Levi C (2012) A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 366(12):1099–1107
Wang Y, Li S, Pan Y, Li H, Parsons MW, Campbell BCV, Schwamm LH, Fisher M, Che F, Dai H, Li D, Li R, Wang J, Wang Y, Zhao X, Li Z, Zheng H, Xiong Y, Meng X, Li R, Wang D, Wang Y, Chen S, Deng D, Zhang H, Wang J, Chen H, Zhang H, Wu Y, Liu H, Lu G, Zhao L, Zhu R, Liu Y, Yi F, Gao J, Dai H, Hao J, Che F, Cai X, Duan Z, Yu H, Wei T, Tang Y, Peng Z, Zhang B, Song Y, Chen X, Liu Y, Liu J, Li D, Zhao W, Wei X, Xue Q, Liu X, Yang Y, Zhao C, Chen J, Sui Y, Sheng G, Zhang Y, Liu J, Zhang L, Wang W, Guo Z, Li H, Hu R, Chen G, Liang Z, Chen J, Xia L, Long Z (2023) Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet 401(10377):645–654
Menon BK, Buck BH, Singh N, Deschaintre Y, Almekhlafi MA, Coutts SB, Thirunavukkarasu S, Khosravani H, Appireddy R, Moreau F, Gubitz G, Tkach A, Catanese L, Dowlatshahi D, Medvedev G, Mandzia J, Pikula A, Shankar J, Williams H, Field TS, Manosalva A, Siddiqui M, Zafar A, Imoukhuede O, Hunter G, Demchuk AM, Mishra S, Gioia LC, Jalini S, Cayer C, Phillips S, Elamin E, Shoamanesh A, Subramaniam S, Kate M, Jacquin G, Camden MC, Benali F, Alhabli I, Bala F, Horn M, Stotts G, Hill MD, Gladstone DJ, Poppe A, Sehgal A, Zhang Q, Lethebe BC, Doram C, Ademola A, Shamy M, Kenney C, Sajobi TT, Swartz RH (2022) Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 400(10347):161–169
Kvistad CE, Næss H, Helleberg BH, Idicula T, Hagberg G, Nordby LM, Jenssen KN, Tobro H, Rörholt DM, Kaur K, Eltoft A, Evensen K, Haasz J, Singaravel G, Fromm A, Thomassen L (2022) Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol 21(6):511–519
Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, Thommessen B, Amthor KF, Ihle-Hansen H, Kurz M, Tobro H, Kaur K, Stankiewicz M, Carlsson M, Morsund Å, Idicula T, Aamodt AH, Lund C, Næss H, Waje-Andreassen U, Thomassen L (2017) Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 16(10):781–788
Burgos AM, Saver JL (2019) Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke 50(8):2156–2162
Thelengana A, Radhakrishnan DM, Prasad M, Kumar A, Prasad K (2019) Tenecteplase versus alteplase in acute ischemic stroke: systematic review and meta-analysis. Acta Neurol Belg 119(3):359–367
Zang Y, Hou J, Wang LY (2016) Therapeutic effect of tenecteplase on treatment of cerebral arterial thrombosis: a meta-analysis. Eur Rev Med Pharmacol Sci 20(20):4369–4379
Qureshi AI, Baskett WI, Bains NK, French BR, Siddiq F, Gomez CR, Shyu CR (2022) Outcomes with IV tenecteplase and IV alteplase for acute ischemic stroke with or without thrombectomy in real-world settings in the United States. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 32(2):106898
Walton MN, Hamilton LA, Salyer S, Wiseman BF, Forster AM, Rowe AS (2023) Major Bleeding Postadministration of Tenecteplase Versus Alteplase in Acute Ischemic Stroke. Ann Pharmacother 57(5):535–543. https://doi.org/10.1177/10600280221120211
Mohan A, Komakula S, Murali S, Anand P, Shah D, Vishnu VY, Pandit AK, Agarwal A, Vibha D, Singh MB, Padma Srivastava MV, Bhatia R (2023) Biosimilar tenecteplase versus alteplase in acute ischemic stroke: a real world study. Ann Indian Acad Neurol 26(1):54–58
Seners P, Caroff J, Chausson N, Turc G, Denier C, Piotin M, Aghasaryan M, Alecu C, Chassin O, Lapergue B, Naggara O, Ferrigno M, Arquizan C, Cho TH, Narata AP, Richard S, Bricout N, Mazighi M, Costalat V, Gory B, Debiais S, Consoli A, Bracard S, Oppenheim C, Mas JL, Smadja D, Spelle L, Baron JC (2019) Recanalization before thrombectomy in tenecteplase vs. alteplase-treated drip-and-ship patients. J Stroke 21(1):105–107
Beharry J, Waters MJ, Drew R, Fink JN, Wilson D, Campbell BCV, Parsons MW, Kleinig TJ, Wu TY (2020) Dabigatran reversal before intravenous tenecteplase in acute ischemic stroke. Stroke 51(5):1616–1619
Warach SJ, Saver JL (2020) Stroke thrombolysis with tenecteplase to reduce emergency department spread of coronavirus disease 2019 and shortages of alteplase. JAMA Neurol 77(10):1203–1204
Warach SJ, Dula AN, Milling TJ, Miller S, Allen L, Zuck ND, Miller C, Jesser CA, Misra LR, Miley JT, Mawla M, Ding MC, Bertelson JA, Tsui AY, Jefferson JR, Davison HM, Shah DN, Ellington KT, Padrick MM, Nova AS, Krishna VR, Davis LA, Paydarfar D (2022) Prospective observational cohort study of tenecteplase versus alteplase in routine clinical practice. Stroke. 53(12):3583–3593. https://doi.org/10.1161/STROKEAHA.122.038950
Teivane A, Jurjans K, Vetra J, Grigorjeva J, Kupcs K, Masiliunas R, Miglane E (2022) Tenecteplase or alteplase better in patients with acute ischemic stroke due to large vessel occlusion: a single center observational study. Medicina (Kaunas) 58(9):1169. https://doi.org/10.3390/medicina58091169
Li S, Pan Y, Wang Z, Liang Z, Chen H, Wang D, Sui Y, Zhao X, Wang Y, Du W, Zheng H, Wang Y (2022) Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol 7(1):47–53
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB, Grp M (2000) Meta-analysis of observational studies in epidemiology—a proposal for reporting. JAMA J Am Med Assoc 283(15):2008–2012
Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz M, Kuijpers T, Meerpohl J, Morgan R, Mustafa R, Skoetz N, Sultan S, Wiysonge C, Guyatt G, Schünemann HJ (2020) GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 119:126–135
Ye Z, Busse JW, Hill MD, Lindsay MP, Guyatt GH, Prasad K, Agarwal A, Beattie C, Beattie J, Dodd C, Heran MKS, Narayan S, Chartúir NN, O’Donnell M, Resmini I, Sacco S, Sylaja PN, Volders D, Wang X, Xie F, Zachrison KS, Zhang L, Zhong H, An Z, Smith EE (2022) Endovascular thrombectomy and intravenous alteplase in patients with acute ischemic stroke due to large vessel occlusion: a clinical practice guideline. J Evid Based Med 15(3):263–271
Kobeissi H, Ghozy S, Turfe B, Bilgin C, Kadirvel R, Kallmes DF, Brinjikji W, Rabinstein AA (2023) Tenecteplase vs. alteplase for treatment of acute ischemic stroke: a systematic review and meta-analysis of randomized trials. Front Neurol 14:1102463
Katsanos AH, Safouris A, Sarraj A, Magoufis G, Leker RR, Khatri P, Cordonnier C, Leys D, Shoamanesh A, Ahmed N, Alexandrov AV, Tsivgoulis G (2021) Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta-analysis. Stroke 52(1):308–312
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernan MA, Hopewell S, Hrobjartsson A, Junqueira DR, Juni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Sterne JAC, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Wetterslev J, Thorlund K, Brok J, Gluud C (2009) Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 9:86
Wetterslev J, Thorlund K, Brok J, Gluud C (2008) Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 61(1):64–75
Brok J, Thorlund K, Gluud C, Wetterslev J (2008) Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 61(8):763–769
Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J (2015) Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ 350:h1354
Beliveau A, Boyne DJ, Slater J, Brenner D, Arora P (2019) BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network meta-analyses. BMC Med Res Methodol 19(1):196
Tonin FS, Rotta I, Mendes AM, Pontarolo R (2017) Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada) 15(1):943
Salanti G, Ades AE, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64(2):163–171
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE (2014) In: NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials. London
Toft N, Innocent GT, Gettinby G, Reid SW (2007) Assessing the convergence of Markov Chain Monte Carlo methods: an example from evaluation of diagnostic tests in absence of a gold standard. Prev Vet Med 79(2–4):244–256
Dias S, Welton NJ, Caldwell DM, Ades AE (2010) Checking consistency in mixed treatment comparison meta-analysis. Stat Med 29(7–8):932–944
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook
Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, Gagnier J, Borenstein M, van der Heijden G, Dahabreh IJ, Sun X, Sauerbrei W, Walsh M, Ioannidis JPA, Thabane L, Guyatt GH (2020) Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 192(32):E901-e906
Mitchell PJ, Yan B, Churilov L, Dowling RJ, Bush SJ, Bivard A, Huo XC, Wang G, Zhang SY, Ton MD, Cordato DJ, Kleinig TJ, Ma H, Chandra RV, Brown H, Campbell BCV, Cheung AK, Steinfort B, Scroop R, Redmond K, Miteff F, Liu Y, Duc DP, Rice H, Parsons MW, Wu TY, Nguyen HT, Donnan GA, Miao ZR, Davis SM (2022) Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet 400(10346):116–125
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650):924–926
Zeng L, Helsingen LM, Bretthauer M, Agoritsas T, Vandvik PO, Mustafa RA, Busse J, Siemieniuk RAC, Lytvyn L, Li SA, Yang M, Yan L, Zhang L, Brignardello-Petersen R, Guyatt GH (2023) A novel framework for incorporating patient values and preferences in making guideline recommendations: guideline panel surveys. J Clin Epidemiol 161:164–172. https://doi.org/10.1016/j.jclinepi.2023.07.003
Zeng L, Li SA, Yang M, Yan L, Helsingen LM, Bretthauer M, Agoritsas T, Vandvik PO, Mustafa RA, Busse J, Siemieniuk RAC, Lytvyn L, Zhang L, Brignardello-Petersen R, Guyatt GH (2023) Qualitative study of guideline panelists: innovative surveys provided valuable insights regarding patient values and preferences. J Clin Epidemiol 161:173–180. https://doi.org/10.1016/j.jclinepi.2023.07.014
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook
Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Thijs V, Scroop R, Simpson M, Brooks M, Asadi H, Wu TY, Shah DG, Wijeratne T, Zhao H, Alemseged F, Ng F, Bailey P, Rice H, de Villiers L, Dewey HM, Choi PMC, Brown H, Redmond K, Leggett D, Fink JN, Collecutt W, Kraemer T, Krause M, Cordato D, Field D, Ma H, O’Brien B, Clissold B, Miteff F, Clissold A, Cloud GC, Bolitho LE, Bonavia L, Bhattacharya A, Wright A, Mamun A, O’Rourke F, Worthington J, Wong AA, Levi CR, Bladin CF, Sharma G, Desmond PM, Parsons MW, Donnan GA, Davis SM (2020) Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK Part 2 randomized clinical trial. JAMA 323(13):1257–1265
Ramakrishnan TCR, Kumaravelu S, Narayan SK, Buddha SS, Murali C, Majeed PHA, Meenakshi-Sundaram S, Wadia RS, Sharma V, Basu I, Vijaya P, Salam KA, Barmare S, Vaid Z, Nirmal Raj KK, Wattamwar PR, Asokan K, Dhonge V, Nellikunja S, Namjoshi D, Srinivasa R, Laddhad DS, Deshpande SD, Raghunath B, Kalita J, Kumar M, Misra UK, Pradeep M (2018) Efficacy and safety of intravenous tenecteplase bolus in acute ischemic stroke: results of two open-label, multicenter trials. Am J Cardiovasc Drugs 18(5):387–395
Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, Ford I, Muir KW (2015) Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol 14(4):368–376
Haley EC Jr, Thompson JL, Grotta JC, Lyden PD, Hemmen TG, Brown DL, Fanale C, Libman R, Kwiatkowski TG, Llinas RH, Levine SR, Johnston KC, Buchsbaum R, Levy G, Levin B (2010) Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke 41(4):707–711
Alemseged F, Ng FC, Williams C, Puetz V, Boulouis G, Kleinig TJ, Rocco A, Wu TY, Shah D, Arba F, Kaiser D, Di Giuliano F, Morotti A, Sallustio F, Dewey HM, Bailey P, O’Brien B, Sharma G, Bush S, Dowling R, Diomedi M, Churilov L, Yan B, Parsons MW, Davis SM, Mitchell PJ, Yassi N, Campbell BCV (2021) Tenecteplase vs alteplase before endovascular therapy in basilar artery occlusion. Neurology 96(9):e1272–e1277
Coutts SB, Dubuc V, Mandzia J, Kenney C, Demchuk AM, Smith EE, Subramaniam S, Goyal M, Patil S, Menon BK, Barber PA, Dowlatshahi D, Field T, Asdaghi N, Camden MC, Hill MD (2015) Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke 46(3):769–774
Dimova SA, Levins ES, Bode ED, Mah ND (2023) Comparison of door-to-needle time of tenecteplase versus alteplase for acute ischemic stroke. Am J Emerg Med 63:158–160. https://doi.org/10.1016/j.ajem.2022.09.030
Estella Á, Pérez Ruiz M, Serrano JJ (2022) Effectiveness and safety of tecneplase vs. alteplase in the acute treatment of ischemic stroke. J Pers Med 12(9):1525
George M, Baby N, Paul R, Zabeer M, Thomas C (2021) Comparison of thrombolytic agents in treatment of patients with acute ischemic stroke; findings from a single centre follow up study in real-life settings. J Clin Neurosci 91:299–305
Gerschenfeld G, Liegey JS, Laborne FX, Yger M, Lyon V, Checkouri T, Tricard-Dessagne B, Marnat G, Clarençon F, Chausson N, Turc G, Sibon I, Alamowitch S, Olindo S (2022) Treatment times, functional outcome, and hemorrhage rates after switching to tenecteplase for stroke thrombolysis: insights from the TETRIS registry. Eur Stroke J 7(4):358–364
Haley EC Jr, Lyden PD, Johnston KC, Hemmen TM (2005) A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke 36(3):607–612
Hendrix P, Collins MK, Griessenauer CJ, Goren O, Melamed I, Weiner GM, Dalal SS, Kole MJ, Noto A, Schirmer CM (2023) Tenecteplase versus alteplase before mechanical thrombectomy: experience from a US healthcare system undergoing a system-wide transition of primary thrombolytic. J Neurointerv Surg 15(e2):e277–e281. https://doi.org/10.1136/jnis-2022-019662
Mahawish K, Gommans J, Kleinig T, Lallu B, Tyson A, Ranta A (2021) Switching to tenecteplase for stroke thrombolysis: real-world experience and outcomes in a Regional Stroke Network. Stroke 52(10):e590–e593
Mathew T, Kile R (2022) Assessing agents for acute stroke. US Pharm 47(10):HS-10-HS−13
Parsons MW, Miteff F, Bateman GA, Spratt N, Loiselle A, Attia J, Levi CR (2009) Acute ischemic stroke: imaging-guided tenecteplase treatment in an extended time window. Neurology 72(10):915–921
Psychogios K, Palaiodimou L, Katsanos AH, Magoufis G, Safouris A, Kargiotis O, Spiliopoulos S, Papageorgiou E, Theodorou A, Voumvourakis K, Broutzos E, Stamboulis E, Tsivgoulis G (2021) Real-world comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke patients with large vessel occlusion. Ther Adv Neurol Disord 14(no pagination)
Sundar K, Bhirud L, Panwar A, Cherian JJ, Paul EM, Kuruttukulam GV (2019) Tenecteplase versus alteplase (TENVALT): a study comparing two thrombolytic agents in acute ischemic stroke. Neurol Asia 24(3):203–208
Tsivgoulis G, Katsanos AH, Christogiannis C, Faouzi B, Mavridis D, Dixit AK, Palaiodimou L, Khurana D, Petruzzellis M, Psychogios K, Macleod MJ, Ahmed N (2022) Intravenous thrombolysis with tenecteplase for the treatment of acute ischemic stroke. Ann Neurol 92(3):349–357
Zhong CS, Beharry J, Salazar D, Smith K, Withington S, Campbell BCV, Wilson D, Le Heron C, Mason D, Duncan R, Reimers J, Mein-Smith F, Diprose WK, Barber PA, Ranta A, Fink JN, Wu TY (2021) Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke 52(3):1087–1090
Dhar N, Kumar M, Tiwari A, Desai I, Madhaw G, Kumar N (2022) Tenecteplase and alteplase for thrombolysis of acute ischemic stroke within 4.5 hours: an efficacy and safety study. Ann Indian Acad Neurol 25(5):897–901
Ray B, Janzen KM, Curran M, Adamson R, Allen L, Warach S, Daley M (2023) Comparison of dosing errors between tenecteplase and alteplase for management of acute ischemic stroke. J Am Pharm Assoc (2003) 63(2):643–647
Dittmar E, Wolfel T, Menendez L, Pozo J, Ramirez M, Belnap SC, De Los Rios La Rosa F (2023) Conversion from intravenous alteplase to tenecteplase for treatment of acute ischemic stroke across a large community hospital health system. Ann Pharmacother 57(10):1147–1153. https://doi.org/10.1177/10600280221149409
Zhang X, Wan TF, Chen J, Liu L (2023) Tenecteplase versus alteplase for patients with acute ischemic stroke: a meta-analysis of randomized controlled trials. Aging (Albany NY) 15(24):14889–14899
Salamatullah HK, Bashrahil B, Alghamdi AM, Alsharm FS, Alkulli OA, Alzahrani Z, Alkhiri A, Alghamdi S, Makkawi S (2023) Efficacy and safety of tenecteplase in comparison to alteplase in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Neurol Neurosurg 233:107961
Liang H, Wang X, Quan X, Chen S, Qin B, Liang S, Huang Q, Zhang J, Liang Z (2023) Different doses of tenecteplase vs. alteplase for acute ischemic stroke within 4.5 hours of symptom onset: a network meta-analysis of randomized controlled trials. Front Neurol 14:1176540
Ma P, Zhang Y, Chang L, Li X, Diao Y, Chang H, Hui L (2022) Tenecteplase vs. alteplase for the treatment of patients with acute ischemic stroke: a systematic review and meta-analysis. J Neurol 269(10):5262–5271
Oliveira M, Fidalgo M, Fontão L, Antão J, Marques S, Afreixo V, Gregório T (2021) Tenecteplase for thrombolysis in stroke patients: systematic review with meta-analysis. Am J Emerg Med 42:31–37
Xiong Y, Wang L, Li G, Yang KX, Hao M, Li S, Pan Y, Wang Y (2023) Tenecteplase versus alteplase for acute ischaemic stroke: a meta-analysis of phase III randomised trials. Stroke Vasc Neurol. https://doi.org/10.1136/svn-2023-002396
Rehman AU, Mohsin A, Cheema HA, Zahid A, Rehman MEU, Ameer MZ, Ayyan M, Ehsan M, Shahid A, Rehman MAU, Shah J, Khawaja A (2023) Comparative efficacy and safety of tenecteplase and alteplase in acute ischemic stroke: a pairwise and network meta-analysis of randomized controlled trials. J Neurol Sci 445:120537
Abuelazm M, Seri AR, Awad AK, Ahmad U, Mahmoud A, Albazee E, Kambalapalli S, Abdelazeem B (2023) The efficacy and safety of tenecteplase versus alteplase for acute ischemic stroke: an updated systematic review, pairwise, and network meta-analysis of randomized controlled trials. J Thromb Thrombolysis 55(2):322–338. https://doi.org/10.1007/s11239-022-02730-5
The EZ, Lin NN, Matar M, Teoh HL, Yeo LLL (2023) Different dosing regimens of Tenecteplase in acute ischemic stroke: A network meta-analysis of the clinical evidence. Eur Stroke J 8(1):93–105. https://doi.org/10.1177/23969873221129924
Kheiri B, Osman M, Abdalla A, Haykal T, Ahmed S, Hassan M, Bachuwa G, Al Qasmi M, Bhatt DL (2018) Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. J Thromb Thrombolysis 46(4):440–450
Verde PE (2021) A bias-corrected meta-analysis model for combining, studies of different types and quality. Biom J 63(2):406–422
Behrouz R (2014) Intravenous tenecteplase in acute ischemic stroke: an updated review. J Neurol 261(6):1069–1072
Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Liang S, Venketasubramanian N, Rathakrishnan R, Ahmad A, Ng KW, Loh PK, Ong JJ, Wakerley BR, Chong VF, Bathla G, Sharma VK (2013) Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol 70(3):353–358
Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, Lai J, Peña L, Pater C, Ogez J et al (1994) A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci USA 91(9):3670–3674
Funding
This study is found by National Natural Science Foundation of China (Grant nos. 82274368 and 72204173), National Science Fund for Distinguished Young Scholars (Grant no. 82225049), Sichuan Provincial Central Government Guides Local Science and Technology Development Special Project (Grant no. 2022ZYD0127), and Fundamental Research Funds for the Central public welfare research institutes (Grant no. 2020YJSZX-3). JWB is supported, in part, by a CIHR Canada Research Chair in Prevention & Management of Chronic Pain.
Author information
Authors and Affiliations
Contributions
Yu Ma and Hunong Xiang contributed equally as co-first authors. Yu Ma and Hunong Xiang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Ling Li, Xin Sun, Yu Ma, Hunong Xiang, Jian Guo and Bo Li designed the study. Yu Ma, Hunong Xiang, Jason W. Busse, Ling Li and Xin Sun drafted the manuscript. Yu Ma, Hunong Xiang and Minghong Yao conducted the statistical analysis. Ling Li, Xin Sun, Jason W. Busse, Minghong Yao, and Long Ge gave administrative, technical, or material support. Xin Sun and Ling Li supervised the study. All authors analyzed and interpreted the data. All authors approved the critical revision of the manuscript for important intellectual content.
Corresponding authors
Ethics declarations
Conflicts of interest
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval
Not required.
Transparency
The manuscript’s guarantors (XS and LL) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Xiang, H., Busse, J.W. et al. Tenecteplase versus alteplase for acute ischemic stroke: a systematic review and meta-analysis of randomized and non-randomized studies. J Neurol 271, 2309–2323 (2024). https://doi.org/10.1007/s00415-024-12243-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12243-1