Abstract
Autoantibodies against contactin-associated protein 2 (Caspr2) not only induce limbic autoimmune encephalitis but are also associated with pain conditions. Here, we analyzed clinical data on pain in a large cohort of patients included into the German Network for Research in Autoimmune Encephalitis. Out of 102 patients in our cohort, pain was a frequent symptom (36% of all patients), often severe (63.6% of the patients with pain) and/or even the major symptom (55.6% of the patients with pain). Pain phenotypes differed between patients. Cluster analysis revealed two major phenotypes including mostly distal-symmetric burning pain and widespread pain with myalgia and cramps. Almost all patients had IgG4 autoantibodies and some additional IgG1, 2, and/or 3 autoantibodies, but IgG subclasses, titers, and presence or absence of intrathecal synthesis were not associated with the occurrence of pain. However, certain pre-existing risk factors for chronic pain like diabetes mellitus, peripheral neuropathy, or preexisting chronic back pain tended to occur more frequently in patients with anti-Caspr2 autoantibodies and pain. Our data show that pain is a relevant symptom in patients with anti-Caspr2 autoantibodies and support the idea of decreased algesic thresholds leading to pain. Testing for anti-Caspr2 autoantibodies needs to be considered in patients with various pain phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoantibodies against contactin-associated protein 2 (Caspr2) are associated with a variety of clinical phenotypes including limbic encephalitis, neuromyotonia, cerebellar dysfunction, dysautonomia, insomnia, movement disorders, and neuropathic pain [1,2,3,4]. The autoantibodies mostly belong to the IgG4 subclass that does neither induce complement deposition, activation of inflammatory cells, nor internalization of surface proteins, but additional IgG1 autoantibodies have also been described [3]. Caspr2 is part of the voltage-gated potassium channel complex that modulates neuronal excitability [5]. Neuropathic pain is supposed to be induced by binding of anti-Caspr2 autoantibodies to dorsal root ganglia (DRG) neurons, resulting in hyper-excitability of nociceptive neurons [6]. However, neuropathic pain is only reported in about 30–60% of all patients with anti-Caspr2 autoantibodies, and the phenotype of pain varies [2, 4, 7,8,9]. Individual differences either of the patients or at the autoantibody level may account for differences in the induction of neuropathic pain.
In the present study, a large multicenter cohort of patients with anti-Caspr2 autoantibodies was screened for neuropathic pain. The clinical phenotypes of patients with and without neuropathic pain as well as IgG subclass distribution and autoantibody titers were systematically assessed and compared.
Methods
Study cohort
The registry of the German Network for Research on Autoimmune Encephalitis (GENERATE), a multicenter database for patients with autoimmune encephalitis in Germany, Austria, and Switzerland (generate-net.de) was searched for patients who had been positively tested for anti-Caspr2 autoantibodies at the participating centers between 2011 and 2023, resulting in 115 datasets of patients. All datasets were manually checked for plausibility. Five patients were finally excluded due to lacking information on pain symptoms, eight were excluded because of very low anti-Caspr2 titers (1:10 or less). Autoantibody testing was performed at different laboratories, all using cell-based assays. The antibody index was calculated according to Reiber et al., a cut-off value of > 4 was applied for intrathecal synthesis [10].
All patients gave written informed consent to be enrolled in the GENERATE registry and the registry was approved by the Ethic committees of all participating centers.
All recruiting centers were contacted and asked for information on pain in patients treated at their hospital. Only patients whose records contained information on pain were finally included into the study, resulting in a study cohort of 102 patients. All centers were asked to retrospectively extract the following information on pain from the patient records: localization, quality, intensity, temporal course, relieving/deteriorating factors, major symptom (yes/no), and response to immunosuppressive treatment (mostly glucocorticoids and/or rituximab). In most hospitals, pain intensity was scaled from 0 to 10 on the numeric rating scale. From some patients, no numeric pain rating was available, and pain was only categorized as “mild”, “moderate”, or “severe” in the patient records. We therefore decided to use these three categories and the values from the numeric rating scale were categorized as mild (1–3), moderate (4–6), or severe (7–10) accordingly. Additionally, information regarding the following conditions was requested: neuromyotonia, peripheral neuropathy, and diabetes mellitus.
Detection of anti-Caspr2 autoantibodies and IgG subclass analysis
Sera for the analysis of IgG subclasses were available from 48 of the included patients. First, the presence of autoantibodies against Caspr2 in the sera was validated via cell-based assays (CBA) using human embryonic kidney 293 (HEK293, (CRL-1573; ATCC – Global Bioresource Center, Manassas, VA, USA) cells. After one day of culturing at 37 °C and 5% CO2, the cells were transfected with the Caspr2 plasmid (kindly provided by J. Dalmau [11]) using calcium phosphate precipitation as described before [12]. After two days, the transfected cells were incubated with patient serum (1:250, one serum that was negative at 1:250 was repeated with a dilution of 1:100) and a commercial anti-Caspr2 antibody from sheep (1:250, R&D Systems by Bio-Techne, AF5145) for one hour. Then, the cells were fixed with 4% PFA and 4% sucrose in PBS (pH 7.4) for 20 min on ice. Blocking with 5% horse serum in PBS (pH 7.4) at room temperature was performed for 30 min. Afterward, the cells were incubated with donkey anti-sheep Alexa Fluor 488 (Jackson ImmunoResearch, 713–545-147) and goat anti-human Cy3 (Jackson ImmunoResearch, 109–165-003) secondary antibodies diluted 1:500 in PBS (pH 7.4) for an hour at room temperature. The coverslips were incubated with DAPI (4’, 6-Diamidino-2-phenylindol, 1:5000) for 5 min and then mounted with Mowiol.
For the IgG subclass determination, the CBA described above was conducted using IgG subclass-specific secondary antibodies. Initially, the cells were exposed to patient serum and serum of healthy controls with a dilution of 1:250. Subclass determination for sera with low titers was exhibited with a 1:50 or the lowest 1:25 dilution of the patient sera. FITC- or Alexa Fluor 488-conjugated commercial secondary antibodies at a dilution of 1:100 were applied (anti-human IgG1: Abcam, ab99772; anti-human IgG2: Southern Biotech, 9070–30; anti-human IgG3: Sigma Aldrich, F4641; anti-human IgG4: Abcam, ab99815). As controls, coverslips with untransfected cells were incubated with serum and goat anti-human Cy3 secondary antibody or stained with commercial anti-Caspr2 primary antibody from sheep and donkey anti-sheep Alexa Fluor 488 as the secondary antibody.
Statistical analysis
All statistical tests were performed using R version 3.4.1. Polytomous Latent Class analysis for clustering was performed using R package poLCA (version 1.6.0).
The motivation for Polytomous Latent Class analysis was that this method allows to identify and estimate if the underlying distribution is a mixture or not. This was achieved using the Akaike Information Criterion (AIC) for model order selection. If the model with the best (minimal) AIC has more than one component, this characterizes clusters of observations coming from the same distribution.
For each number of clusters, the minimum AIC of 25 runs was calculated. Optimal number of components was determined by the minimum AIC.
Cluster analysis included the following dichotomous variables: distal > proximal pain, back pain, myalgia/arthralgia, high intensity, increase with exercise, burning pain, muscle soreness, cramps, tearing pain, dysesthesia, dull pain, temperature-dependent pain, pain as major symptom, no response to treatment. For comparison of metric data, t tests were performed, for categorical data, chi-square test was used, and a significance level of < 0.05 was applied in all tests.
Results
Patient cohort
Of the 102 anti-Caspr2-positive patients who were finally included, 37 reported chronic pain, 65 did not report any pain (Fig. 1). Only pain that occurred for the first time at the onset of anti-Caspr2-associated disease or clearly exacerbated in temporal relation with the disease was considered, no other chronic pain states. Median age, sex and autoantibody titers are summarized in Table 1 (left two columns) and did not differ between both groups.
78 of the patients (51 without pain, 27 with pain) had symptoms of limbic encephalitis and fulfilled the clinical diagnostic criteria of possible autoimmune encephalitis according to Graus et al. [13] (and of course definite autoimmune encephalitis when considering the positive anti-Caspr2 findings). The others did not show any cognitive, mental, or psychiatric symptoms but other symptoms indicating anti-Caspr2-associated disease like cerebellar dysfunction, seizures, neuromyotonia, or autonomic symptoms.
Autoantibody titers and distribution
Serum and cerebrospinal fluid (CSF) titers of diagnostic anti-Caspr2 testing were retrieved from the GENERATE database or the patients records and were available in 48 patients without pain and 37 patients with pain, all tested by assays from Euroimmun (Lübeck, Germany). The remaining 17 patients were clearly positive for anti-Caspr2 with other tests and/or the initial titer could not be reported by the recruiting center. Of the patients without pain, 37/48 (77.1%) were anti-Caspr2-positive in the serum and CSF, 10/48 (20.8%) were only positive in the serum and 1/48 (2.1%) only in the CSF. Of the anti-Caspr2-positive patients with pain, 21/37 (56.8%) were positive in the serum and CSF, 9/37 (24.3%) were only positive in the serum and none of the patients only in the CSF. In seven patients, no information of CSF titer was available. Titers did not differ between patients with and without pain (Table 1, left two columns). The number of patients with CSF pleocytosis was also similar in patients with and without pain (even if there was a slight trend toward more patients with pleocytosis in the cohort without pain (Table 1)). The median time between symptom onset and lumbar puncture was 4 months in both groups (range 0–197 months (without pain) and 0–106 (with pain)) and did not differ between groups. There was no correlation between pleocytosis and the time between symptom onset and CSF analysis. Intrathecal autoantibody production (based on the autoantibody index) could be assessed in 43 patients without pain and 30 patients with pain. Intrathecal production could be found in 23/43 (53.5%) of the patients without pain and 16/30 (53.3%) with pain. Thus, neither the autoantibody titers nor the distribution of autoantibodies between compartments (CSF vs. serum) could be clearly attributed to painful or painless manifestations.
IgG subclasses in patients with/without pain
IgG subclasses of anti-Caspr2 could be determined in sera of 40 patients, 21 patients without pain and 19 patients with pain (Fig. 2A, B, Table 1 left two columns). In sera of eight patients (all with low titers of anti-Caspr2), no subclass was detectable, i.e., all CBA with subclass-specific secondary antibodies were negative, most probably because of the low titer.
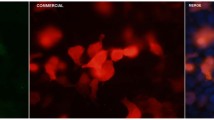
Patient anti-Caspr2 autoantibodies show distinct IgG subclass pattern independent of the pain phenotype. HEK293 cells were transfected with human Caspr2. A Caspr2 was stained with a commercial antibody (1:250; cyan; upper left image). Secondary FITC-coupled IgG subclass antibodies (IgG1-4) were tested with healthy control (HC, 1:250) serum instead of patient (pat) serum as negative controls (upper lane, right images). Representative images following incubation with human patient serum 13 (1:250) detected by secondary antibodies against total human IgG (red; lower left image). Serum of patient 13 (1:50 for subclass determination) was positive for all four IgG subclasses but with different intensities (IgG3 < IgG1 < IgG2 = IgG4; cyan, lower right images). DAPI marks the nuclei. Scale bar refers to 10 µm. B Intensity plot of IgG subclass determination from all patients (40 total) investigated. Intensities were classified from 0–1-2–3 (white—light blue—blue—dark blue; no staining—very weak but visible staining—intense staining—very intense staining). Note, almost all sera contained Caspr2 autoantibodies of subclass IgG4. In (C), the probability of different variables of cluster analysis in cluster 1 and 2 is depicted. Patients of cluster 1 suffer from mostly distal burning and tingling pain that is increased by exercise and cold whereas cluster 2 is characterized by widespread pain, myalgia and cramps. pat patient, HC healthy control
IgG4 autoantibodies were detectable in all sera except for two patients (both without pain) where only IgG3 anti-Caspr2 autoantibodies could be found and one serum of a patient with pain where only IgG1 was detectable. In 6/21 (28.6%) sera of patients without pain, IgG4 was the only autoantibody subclass, in 13/21 (61.9%), IgG4, and autoantibodies of other subclasses (IgG1, IgG2 and/or IgG3) were detectable. Of the patients with pain, 4/19 (21.1%) had IgG4 autoantibodies only, in 14/19 (73.7%) additional other subclasses were detectable.
Two major pain phenotypes associated with anti-Caspr2 autoantibodies
In the patients with anti-Caspr2 autoantibodies and pain, information on the severity of pain was available in 22 patients and was reported as “severe” by most of them (14/22), as “moderate” by 6/22 and as mild by only two patients. Pain was the major symptom in 20/37 patients who experienced pain and the only symptom in two patients. When evaluating the descriptions of the pain phenotypes of the anti-Caspr2-positive patients, different phenotypes became apparent: Some patients suffered from distal-symmetric burning pain and/or allodynia, corresponding to the pain phenotype typically found in patients with small fiber neuropathy. Other patients experienced severe back pain radiating to the legs, resembling radiculitis and other patients reported chronic widespread pain including myalgia and arthralgia, in some cases accompanied by muscle cramps.
To better differentiate pain phenotypes, cluster analysis including localization, severity, provoking factors, and clinical description of pain was performed using model order selection. Four clusters were proposed, but analysis was adapted to two clusters due to plausibility and because otherwise the groups would have been too small for further comparison. In cluster 1 (n = 21), pain was located at the distal legs and feet and/or in the back, was often burning or tingling, sometimes relieved by cooling (or aggravated by heat) and was often difficult to treat, thus resembling neuropathic pain experienced in small fiber neuropathy (see Table 2, Fig. 2C). Patients of cluster 2 (n = 16) reported more widespread pain, often myalgia and/or muscle cramps that often responded to treatment (Table 2). Age, sex, autoantibody titers, CSF findings, and IgG subclasses were not different between the clusters (Table 1, right two columns).
Possible risk factors for the development of pain
As IgG subclasses, titers and intra-/extrathecal distribution of autoantibodies did not seem to be associated with the development of pain, we compared the occurrence of comorbidities within groups to identify potential risk factors (see also Table 1). The prevalence of diabetes mellitus was similar in the patients without and with pain (15.4% vs. 16.2%). There was a trend for a higher prevalence of diabetes mellitus in cluster 1 (cluster 1: 23.8% vs. cluster 2: 6.2%), but statistical comparison did not reach significance. Peripheral neuropathies were more often found in patients with painful conditions, especially in those of cluster 1 (painless vs. painful: p = 0.01, cluster 1 vs cluster 2: p = 0.1). Neuromyotonia as another symptom of hyper-excitability was also more prevalent in patients with pain, but without any difference in the two clusters (painless vs. painful: p = 0.0003). A history of chronic back pain and/or spinal surgery was reported by 8/65 (12.3%) patients without pain and 9/37 (24.3%) patients with pain pointing to a trend of a higher prevalence in patients with pain, but the difference was not significant.
Discussion
Assessment and characterization of painful states in patients with anti-Caspr2 autoantibodies that were included in the GENERATE database showed that pain is a relevant symptom in a large proportion of patients, is often severe and may be the major symptom. The pain phenotype differed between patients. However, two patterns could be observed: distal-symmetric burning or radiating back pain and widespread pain, often localized in the muscles associated with cramps. We did not find any association with autoantibody-related factors like titer, IgG subclass, or intrathecal autoantibody synthesis. Instead, certain potential risk factors for chronic pain like peripheral neuropathy, or preexistent chronic back pain as well as neuromyotonia tended to occur more frequently in patients with anti-Caspr2 autoantibodies and pain, arguing in favor of hyper-excitability, preexisting sensitization and/or decreased pain thresholds to play a role in pain induction.
The prevalence of pain in our study cohort is consistent with previous studies that also reported neuropathic pain in 30 to 60% of adult patients and often severe intensity of pain [2, 4, 7, 8, 14]. Painful phenotypes were not associated with different titers or certain IgG subclasses. Thus, there were no evident differences on the autoantibody level that could explain different clinical phenotypes. In contrast to recent studies, that had reported that in patients with neuropathic pain, autoantibodies are only found in the serum whereas in patients with a CNS involvement they are also positive in the CSF, we could not find any differences between patients with and without intrathecal synthesis of autoantibodies and also no association to CSF pleocytosis. This may be explained by the fact that all our patients also had CNS symptoms.
Existing data on the clinical phenotype of pain are rare and may be biased as several studies only included neuropathic pain. Hence, pain states that are not clearly neuropathic (like arthralgia or myalgia) may not have been included: A systematic study using pain questionnaires depicted variable pain qualities and locations, but no uniform phenotypes [7]. Other more descriptive studies and case series often described distal burning pain (as also reported by a relevant proportion of the patients in our cohort) but also back pain, muscle pain and in a pediatric cohort even abdominal pain [3, 8, 9, 15]. Thus, variable pain phenotypes occur in patients with anti-Caspr2 autoantibodies and from a purely clinical point of view do not even clearly correspond to neuropathic pain. From a pathophysiological point of view, regarding hyper-excitability of nociceptive neurons, i.e., peripheral sensitization to pain, as the cause of these pain conditions, pain in patients with anti-Caspr2 autoantibodies may better fit into the newer category of nociplastic pain that was introduced by the International Association for the Study of Pain to differentiate pain with altered nociception from pain that is caused by a distinct lesion to the nervous system [16]. Indeed, the existence of chronic widespread pain and hypersensitivity may argue in favor of this classification [17].
As neither presence or absence of pain nor the pain phenotypes in our study was related to autoantibody titers, IgG subclasses, or intra-/extrathecal autoantibody synthesis, we searched for patient-related factors that may explain these differences, i.e., risk factors for certain pain conditions that may be symptomatic due to peripheral sensitization by autoantibody binding. By clustering patients into two patterns of pain phenotypes, we noted that neuromyotonia appeared to be most frequent in patients with myalgia, whereas a preexisting peripheral neuropathy and diabetes mellitus appeared to be associated with distal-symmetric “small-fiber-neuropathy-like” phenotype. However, larger cohorts of these subgroups are needed to definitely establish risk factors.
In summary, our data confirm pain to be a relevant symptom in patients with anti-Caspr2 autoantibodies, which may be the major or even only symptom and suggest a broad range of pain conditions that can be categorized into two clusters but cannot be defined by IgG subclasses, titers, or CSF/serum positivity. Prospective studies on large cohorts using prespecified pain questionnaires and systematic screening for risk factors are needed to further support our findings. Testing for anti-Caspr2 should be considered in patients with unclear pain, not only in unambiguously neuropathic pain description but also in patients with widespread muscle pain, particularly in combination with muscle cramps.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Govert F, Abrante L, Becktepe J, Balint B, Ganos C, Hofstadt-van Oy U, Krogias C, Varley J, Irani SR, Paneva S, Titulaer MJ, de Vries JM, Boon AJW, Schreurs MWJ, Joubert B, Honnorat J, Vogrig A, Arino H, Sabater L, Dalmau J, Scotton S, Jacob S, Melzer N, Bien CG, Geis C, Lewerenz J, Pruss H, Wandinger KP, Deuschl G, Leypoldt F (2023) Distinct movement disorders in contactin-associated-protein-like-2 antibody-associated autoimmune encephalitis. Brain 146:657–667. https://doi.org/10.1093/brain/awac276
Gadoth A, Pittock SJ, Dubey D, McKeon A, Britton JW, Schmeling JE, Smith A, Kotsenas AL, Watson RE, Lachance DH, Flanagan EP, Lennon VA, Klein CJ (2017) Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG positive patients. Ann Neurol. https://doi.org/10.1002/ana.24979
van Sonderen A, Arino H, Petit-Pedrol M, Leypoldt F, Kortvelyessy P, Wandinger KP, Lancaster E, Wirtz PW, Schreurs MW, Sillevis Smitt PA, Graus F, Dalmau J, Titulaer MJ (2016) The clinical spectrum of Caspr2 antibody-associated disease. Neurology 87:521–528. https://doi.org/10.1212/WNL.0000000000002917
Benoit J, Muniz-Castrillo S, Vogrig A, Farina A, Pinto AL, Picard G, Rogemond V, Guery D, Alentorn A, Psimaras D, Rheims S, Honnorat J, Joubert B (2023) Early-stage contactin-associated protein-like 2 limbic encephalitis: clues for diagnosis. Neurology(R) Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000200041
Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E (1999) Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 24:1037–1047. https://doi.org/10.1016/s0896-6273(00)81049-1
Dawes JM, Weir GA, Middleton SJ, Patel R, Chisholm KI, Pettingill P, Peck LJ, Sheridan J, Shakir A, Jacobson L, Gutierrez-Mecinas M, Galino J, Walcher J, Kuhnemund J, Kuehn H, Sanna MD, Lang B, Clark AJ, Themistocleous AC, Iwagaki N, West SJ, Werynska K, Carroll L, Trendafilova T, Menassa DA, Giannoccaro MP, Coutinho E, Cervellini I, Tewari D, Buckley C, Leite MI, Wildner H, Zeilhofer HU, Peles E, Todd AJ, McMahon SB, Dickenson AH, Lewin GR, Vincent A, Bennett DL (2018) Immune or genetic-mediated disruption of CASPR2 causes pain hypersensitivity due to enhanced primary afferent excitability. Neuron 97(806–822):e10. https://doi.org/10.1016/j.neuron.2018.01.033
Vincent A, Pettingill P, Pettingill R, Lang B, Birch R, Waters P, Irani SR, Buckley C, Watanabe O, Arimura K, Kiernan MC (2018) Association of leucine-rich glioma inactivated protein 1, contactin-associated protein 2, and contactin 2 antibodies with clinical features and patient-reported pain in acquired neuromyotonia. JAMA Neurol 75:1519–1527. https://doi.org/10.1001/jamaneurol.2018.2681
Lahoria R, Pittock SJ, Gadoth A, Engelstad JK, Lennon VA, Klein CJ (2017) Clinical-pathologic correlations in voltage-gated Kv1 potassium channel complex-subtyped autoimmune painful polyneuropathy. Muscle Nerve 55:520–525. https://doi.org/10.1002/mus.25371
Ellwardt E, Geber C, Lotz J, Birklein F (2020) Heterogeneous presentation of caspr2 antibody-associated peripheral neuropathy—a case series. Eur J Pain 24:1411–1418. https://doi.org/10.1002/ejp.1572
Reiber H, Lange P (1991) Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem 37:1153–1160
Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J (2010) Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 9:776–785. https://doi.org/10.1016/S1474-4422(10)70137-X
Chen C, Okayama H (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745–2752
Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Hoftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Pruss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostasy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15:391–404. https://doi.org/10.1016/S1474-4422(15)00401-9
Ramanathan S, Tseng M, Davies AJ, Uy CE, Paneva S, Mgbachi VC, Michael S, Varley JA, Binks S, Themistocleous AC, Fehmi J, Anziska Y, Soni A, Hofer M, Waters P, Brilot F, Dale RC, Dawes J, Rinaldi S, Bennett DL, Irani SR (2021) Leucine-rich glioma-inactivated 1 versus contactin-associated protein-like 2 antibody neuropathic pain: clinical and biological comparisons. Ann Neurol 90:683–690. https://doi.org/10.1002/ana.26189
Syrbe S, Stettner GM, Bally J, Borggraefe I, Bien CI, Ferfoglia RI, Huppke P, Kern J, Polster T, Probst-Muller E, Schmid S, Steinfeld R, Strozzi S, Weichselbaum A, Weitz M, Ziegler A, Wandinger KP, Leypoldt F, Bien CG (2020) CASPR2 autoimmunity in children expanding to mild encephalopathy with hypertension. Neurology 94:e2290–e2301. https://doi.org/10.1212/WNL.0000000000009523
Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, Rief W, Sluka AK (2016) Do we need a third mechanistic descriptor for chronic pain states? Pain 157:1382–1386. https://doi.org/10.1097/j.pain.0000000000000507
Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, Mico JA, Rice ASC, Sterling M (2021) Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain 162:2629–2634. https://doi.org/10.1097/j.pain.0000000000002324
N.D.S.a.t.R.K. Institute (2019) Diabetes in Germany – National Diabetes Surveillance Report 2019. Robert Koch Institute, Berlin
Hanewinckel R, Drenthen J, van Oijen M, Hofman A, van Doorn PA, Ikram MA (2016) Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology 87:1892–1898. https://doi.org/10.1212/WNL.0000000000003293
von der Lippe E, Krause L, Porst M, Wengler A, Leddin J, Muller A, Zeisler ML, Anton A, Rommel A (2021) Prevalence of back and neck pain in Germany. Results from the BURDEN 2020 Burden of Disease Study. J Health Monit 6:2–14. https://doi.org/10.25646/7855
Acknowledgments
We would like to thank Christine Schmitt for excellent technical assistance and Carsten Henneges for help with statistical analysis. Caspr2 plasmids were kindly provided by J. Dalmau. The study was funded by the German Research Foundation, Clinical Research Group “ResolvePain” (KFO5001).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: KD, CV; Methodology: KD, CV, PG, MP; Formal analysis and investigation: KD, CV, PG, MP; Writing—original draft preparation KD, CV, PG; Writing—review and editing: CS, HR, CGB, JL; Funding acquisition: KD, CV, CS, HR; Resources: CB-B, CGB, KE, CG, RH, JK, PK, SK, AK, JL, MM, MN, FvP, HP, AR, JR, SR, RR, TS-H, KS, OS, K-WS, SCT, FT, JW, JW, FL; Supervision: KD, CV.
Corresponding author
Ethics declarations
Conflicts of interest
This study was funded by the German Research Foundation, Clinical Research Group “ResolvePain” (KFO5001). CG received speaker and consultancy fees or travel compensation from Alexion, Sobi, Argenx. His research including the submitted work is funded by the German Federal Ministry of Education and Research (BMBF) through a grant for the Forschungsverbund CONNECT-GENERATE, grant code 01GM2208E, and outside the submitted work by the German Research Foundation and the Schilling foundation. JL received speaker fees or travel compensation from Roche, Teva, the Movement Disorders Society and the Cure Huntington’s Disease Initiative (CHDI). His institution has been reimbursed for his role as principal investigator in trials for SOM Biotech and CHDI. His research including the submitted work is funded by the German Federal Ministry of Education and Research (BMBF) through a grant for the Forschungsverbund CONNECT-GENERATE, grant code 01GM2208B, and outside the submitted work by the European Huntington’s Disease Network (EHDN) and Ministry for Education and Research Baden-Württemberg. FvP reports personal fees and grants from Bial, Desitin Arzneimittel, Eisai, GW Pharmaceutical companies, Arvelle Therapeutics, Zogenix, and UCB Pharma. HR received consultancy fees from Orion and Gruenenthal.
Ethics approval
Ethical approval for the inclusion of patient data to the GENERATE database was waived by the local Ethics Committees of all participating centers. Data were analyzed retrospectively, and data collection and all procedures were part of the routine care.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Greguletz, P., Plötz, M., Baade-Büttner, C. et al. Different pain phenotypes are associated with anti-Caspr2 autoantibodies. J Neurol 271, 2736–2744 (2024). https://doi.org/10.1007/s00415-024-12224-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12224-4