Abstract
Background
Calcitonin gene-related peptide (CGRP) plays an important role in migraine pathophysiology, and post-traumatic headache (PTH) frequently presents with migraine-like features. Despite several clinical similarities, few studies have explored CGRP in PTH and concussion. This study investigates serum CGRP levels in patients with persistent post-concussion symptoms (PPCS), including PTH.
Methods
This cohort study was based on serum samples from individuals aged 18–30 years with PPCS who participated in a previously published randomized controlled trial of a non-pharmacological intervention. The primary outcome was serum CGRP concentrations, determined at baseline before randomization and at follow-up 7 months later, using an enzyme-linked immunosorbent assay (ELISA). CGRP levels at baseline were compared with healthy anonymous blood donors in the same age group.
Results
Baseline serum samples were collected from 86 participants with PPCS. The participants were most often female (78%) and migraine-like headache was the most frequent headache phenotype (74%). Serum CGRP levels were higher in participants with PPCS than in 120 healthy individuals (median: 158.5 pg/mL vs. 76.3 pg/mL, p = 0.050). A stratified analysis revealed that females with PPCS had a fivefold higher median than healthy females (166.3 pg/mL vs. 32.1 pg/mL, p = 0.0006), while no differences were observed in males (p = 0.83). At follow-up, CGRP levels decreased with a median change of – 1.3 pg/mL (95% confidence interval: – 17.6–0, p = 0.024).
Discussion
Elevated serum levels of CGRP in patients with PPCS and a decrease over time suggest an involvement of CGRP in PTH/PPCS. If confirmed in other studies, it could pave the way for CGRP-targeted therapies, which could have clinical significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Concussion is a prevalent injury accounting for up to 98% of all traumatic brain injuries (TBI) [1], and is often referred to as mild TBI [2]. In Europe, the incidence rate of TBI is approximately 300 cases per 100,000 person-years [3], whereas in New Zealand it may be even higher with 790 cases per 100,000 person-years [4]. These incidence rates result in an estimated 55.9 million concussions occurring globally each year [5]. While the majority of those affected recover, up to 30% develop persistent post-concussion symptoms (PPCS) [6] with post-traumatic headache (PTH) being one of the most frequent symptoms [7]. Treating post-traumatic headache (PTH) is a challenge due to the lack of an evidence-based treatment strategy [8], and despite its high prevalence, the underlying pathophysiology remains unknown. Current proposed management guidelines are based on the headache phenotype of PTH [9], which is often migraine-like [10].
During the last 30 years, calcitonin gene-related peptide (CGRP) has substantially increased the understanding of migraine pathophysiology. Initial research revealed increased plasma CGRP levels in migraine patients during attacks [11], and later, its headache-inducing properties were discovered [12]. The importance of CGRP is underlined by CGRP antibodies being used as preventive medication in episodic and chronic migraine. Although the precise pathophysiological mechanism of CGRP remains to be elucidated, it may contribute to migraine due to its ability to cause both peripheral and central sensitization within the trigeminovascular system [13].
Despite the clinical similarities between PTH and migraine, remarkably few studies have examined CGRP concentrations in individuals with concussion who develop PPCS/PTH. One of the few studies available in this field found lower blood CGRP levels in PTH, contrasting with the findings of migraine studies [14]. Due to the paucity of evidence, we aimed to replicate this finding by assessing serum CGRP levels in patients with PPCS/PTH 2–6 months following a concussion.
Methods
Design
This cohort study was based on serum samples from participants included in a recently published open-label parallel-group randomized controlled trial (RCT) conducted at Hammel Neurorehabilitation Centre and University Research Clinic and The Research Clinic for Functional Disorders and Psychosomatics, Aarhus University Hospital in Denmark. The RCT study showed that a new interdisciplinary intervention, Get going After concussIoN (GAIN), significantly lowered PPCS symptoms compared with enhanced usual care (EUC) [15]. The 8-week interdisciplinary intervention was non-pharmacological, based on cognitive behavioral therapy and gradual return to activities, and included both group sessions and individual sessions with a physio/occupational therapist and a neuropsychologist [15]. As outlined in the preregistered analysis plan (referenced in the ‘Ethics Approval and Registrations’ section), we did not anticipate any influence of the non-pharmacological intervention on CGRP levels within the cohort. Consequently, both intervention arms were combined at follow-up akin to a cohort study for the primary outcome.
Participants and inclusion/exclusion criteria
Participants were recruited between March 2015 to September 2017 from general practitioners and hospitals in the Central Denmark Region. Eligible patients were 18–30 years old and had experienced PPCS for 2–6 months. Concussion was defined according to the recommendations from the World Health Organization (WHO) Task Force [16]. Additionally, a direct head trauma was required to rule out pure acceleration-deceleration traumas. PPCS were defined as having a Rivermead Post-Concussion Questionnaire (RPQ) [17] score ≥ 20. Patients with severe brain injury, previous concussion with symptoms > 3 months, drug/alcohol abuse, severe somatic or psychiatric conditions that impeded participation in the intervention, and patients who could not speak Danish were excluded. The inclusion/exclusion criteria were thus identical to those of the previously published RCT [15], except that patients below 18 years of age were not included in this study.
Assessment for eligibility
Potential PPCS patients referred from general practitioners or hospitals were invited to Hammel Neurorehabilitation Centre and University Research Clinic by mail. A thorough assessment for eligibility was done by a neurologist and a psychiatrist and included:
-
(1)
A baseline questionnaire assessing the RPQ score, headache characteristics and symptom burden (see section regarding questionnaire data).
-
(2)
A neurological examination
-
(3)
A standardized psychiatric interview
Further details regarding the assessment procedure have been reported previously [18].
Inclusion of controls
In 2022, we recruited a random cross-sectional sample of healthy, anonymous individuals aged 18–30 from the Blood Bank at Aarhus University Hospital, Denmark, matching the age group of the PPCS participants. The inclusion of healthy individuals allowed us to assess whether CGRP levels were altered in the PPCS participants at baseline, a crucial part of the primary outcome (outlined below). The healthy individuals did not participate in the RCT intervention, and the only demographic data available were age and sex. A sample size of 120 yielded a statistical power of 82% based on the effect size and standard deviations in a study in PTH [14].
Serum samples
The blood samples were drawn from the antecubital vein using serum tubes (Vacutainer Cat 368815 or Cat 367896) and were allowed to clot for 30 min at room temperature. Subsequently, the blood samples were centrifuged for 10 min at 2880 g at 20 °C. The serum was then collected and initially frozen to – 20 °C, and within 24 h moved to – 80 °C until analysis. The samples were stored in 1.5 mL polypropylene tubes (Sarstedt, Nümbrecht, Germany). The preanalytical methodology was the same in samples from healthy individuals and PPCS participants, except the latter samples underwent one freeze–thaw cycle due to the measurement of other biomarkers for another study (not yet published, ClinicalTrials.gov NCT05812742). No protease inhibitors were added, and all participants were non-fasting.
Primary outcome
The primary outcome was serum CGRP concentrations at baseline and follow-up. The baseline blood samples were obtained in connection with the assessment of eligibility 2–6 months after the concussion. The follow-up samples were obtained approximately 7 months later after the completion of the intervention/reference treatment. In contrast, the samples from the anonymous healthy individuals (blood donors) were obtained during their routine voluntary blood donations and no follow-up samples were collected.
Questionnaire data/patient-reported outcomes
Headache data were retrieved from a previously published headache phenotype study conducted in the same PPCS participants [19]. In brief, the PPCS participants filled out a comprehensive headache questionnaire in connection with the assessment of eligibility. It contained data on monthly headache days, duration, current headache pain measured on the visual analogue scale (VAS), questionnaire data on the Headache Impact Test (HIT-6) [20], and allowed classification according to The International Classification of Headache Disorders 3rd edition [21]. Furthermore, questionnaire data on the RPQ, the Short Form (36) Health Survey (SF-36) [22], the Bodily Distress Syndrome Checklist (BDS) [23], Whiteley-8 (WI-8) [24], the “limiting behavior” and “all-or-nothing behavior” subscales from The Behavioural Responses to Illness Questionnaire (BRIQ) [25], The Brief Illness Perception Questionnaire (B-IPQ) [26], Symptom Checklist 8 AD (SCL-8AD) [27], and the Perceived Stress Scale (PSS) [28] were obtained from the RCT-study [15]. Supplementary Table 1 provides an overview of each questionnaire.
CGRP assay
We analyzed the serum samples using a commercial enzyme-linked immunosorbent assay (ELISA) from Bertin Bioreagent which targeted α-CGRP and β-CGRP [29] (Cat No: A05481, Montigny le Bretonneux, France, batch no. 121 & 123). To reduce matrix effects, all calibration curves and quality controls (QCs) were prepared in CGRP-free serum. We prepared CGRP-free serum by incubating a pool of samples from healthy individuals and PPCS participants with CGRP antibodies (Cat No: A19482, Bertin Bioreagent, batch no. 0222) overnight at 4 °C on a tilt shaker. This was then filtered in accordance with Bertin’s instructions. Analysis of three replicates of the resulting CGRP-free serum, using the same methodology as for the study samples, revealed that the depletion was successful (< 2 pg/mL). To assess freeze–thaw stability, we spiked CGRP-depleted serum with 125 pg/mL using the provided CGRP standard in the kit, followed by freezing at – 80 °C. The CGRP concentration remained stable averaging 141.3 pg/mL (SD: 25.4) for three freshly spiked replicates at 125 pg/mL, compared with 122.9 pg/mL (SD: 10.4) after one freeze–thaw-cycle. The observed difference was thus well below the < 20% deviation criterion specified in method validation guidelines [30].
Subsequently, the study samples were thawed at room temperature and analyzed using a calibration curve (7.8–1000 pg/mL) and quality control (125 pg/mL), both freshly prepared in CGRP-free serum. The assay procedure was performed following the manufacturer’s instructions. In short, after loading the samples, the tracer was added, and the plate was incubated overnight (16–20 h at 4 °C). Subsequently, Ellman’s reagent was added, and the resulting color intensity was analyzed with a microplate spectrophotometer (Synergy H1, BioTek, Winooski, The United States (USA)) at 410 nm after 75 min of incubation. Samples from patients and healthy individuals were included on each plate in an equal ratio to ensure representation of both groups. The position of the samples was varied between plates to reduce any bias from plate position. Analyses were conducted by the same experienced bioanalytical technician who was unblinded to the sample group (healthy or PPCS), but was blinded to sex and the hypothesis of the study. A calibration curve was included on each plate and plotted using Graphpad Prism (Graphpad Software, San Diego, USA) from which data were interpolated. The best fit was obtained using a second order polynomial regression (R2 ≥ 0.99, Suppl. Figure 1). CGRP values below limit of detection were replaced with the limit of detection reported by the manufacturer (2 pg/mL), and similarly, values exceeding the detection range were replaced with the highest value of the calibration curve (1000 pg/mL). Dilution of samples exceeding 1000 pg/mL was not performed due to insufficient sample material.
The mean QC concentration on all eight plates was 177.2 pg/mL (SD: 27.3) which was higher than the nominal concentration (125 pg/mL). However, the QCs were highly reproducible with an inter-assay coefficient of variation (CV) of 15.4% and an intra-assay CV ranging from 0% to 13% (median: 6.0%).
Statistical analysis
Since the data were not normally distributed (Suppl. Figure 1), non-parametric tests were employed. CGRP concentration differences between PPCS participants and healthy individuals were assessed with the Wilcoxon rank-sum test, and adjustment for gender was done by stratified analyses. Changes at follow-up in PPCS participants were analyzed with the Wilcoxon signed-rank test. Non-parametric 95% confidence intervals (CI) were generated by bootstrapping the median CGRP difference, computing the resulting 2.5th and 97.5th percentiles across 10,000 replicates. For the primary outcomes, the significance level was set at p ≤ 0.050.
The effect of the non-pharmacological intervention on delta CGRP values (follow-up minus baseline) was evaluated by comparing the delta CGRP values in the treatment arms using the Wilcoxon rank-sum test. This was not included as a primary outcome, since we did not expect the intervention to alter CGRP levels, which was stated in the preregistered analysis plan.
In an exploratory analysis, we correlated serum CGRP levels with headache days, headache duration and current headache pain (VAS score) at baseline using Spearman’s rank correlation. Kruskal-Wallis test was used to test serum CGRP differences between headache phenotypes. Additionally, we assessed the correlation of CGRP levels to patient-reported outcomes (RPQ, HIT-6, SF-36, BDS, WI-8, BRIQ, B-IPQ, SCL-8AD, and PSS) using Spearman’s rank correlation. Finally, we assessed the correlation between delta CGRP and the delta value of the questionnaire data (follow-up minus baseline) using Spearman’s rank correlation. In total, 40 analyses were planned in the exploratory analyses, and the significance level was thus adjusted to p = 0.05/40 = 0.0013 (Bonferroni correction).
All statistical analyses were two-tailed and conducted using Stata 17 for Windows (StataCorp, College Station, USA), and graphs were created using Graphpad Prism.
Results
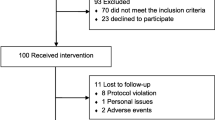
Figure 1 depicts the inclusion procedure and shows that the majority (86 of 112) of the participants from the RCT provided blood samples at baseline at a median of 3.9 months after the trauma (IQR: 3.2–4.7). Most of the participants were female (78%), were on sick-leave (55%), and experienced a concussion in a traffic accident (31%) (Table 1). PTH was highly prevalent in the study as evidenced by 83 of 86 participants reporting headache. The typical participant had a constant headache (38%), 15–31 headache days a month (63%), and had a mixed phenotype between migraine-like and tension-type headache (41%), followed by migraine-like headache alone (34%) (Table 2). Follow-up blood samples were provided by 13 male and 41 female PPCS participants. Serum samples from healthy individuals were collected from 60 anonymous females with a mean age of 25.2 (SD: 2.8) and 60 anonymous males with a mean age of 26.0 (SD: 2.3).
Flow-chart of inclusion procedure. The first blood sample was collected at a median of 4 months after the concussion at baseline of the RCT study before randomization. The second blood sample was obtained 7 months later, at approximately a median of 11 months after the trauma. For baseline comparisons, blood samples from a healthy cross-sectional group of blood donors were included, and these individuals did not participate in the intervention. PPCS Persistent post-concussion symptoms, GP General practitioner, RCT Randomized controlled trial, GAIN acronym for the intervention, Get going After concussIoN (novel non-pharmacological intervention), EUC Enhanced usual care (reference treatment)
Primary/secondary outcomes
Serum CGRP concentrations in PPCS participants at baseline of the RCT study and the healthy individuals are presented in Fig. 2 and Table 3. Median serum CGRP concentrations were doubled in PPCS participants compared to healthy individuals, with a median difference of 82.2 pg/mL (95% CI 17.7–145.6, p = 0.050). When stratifying for sex, it became evident that this difference was due to females with PPCS having a five times higher median concentration than healthy females (166.3 pg/mL vs. 32.1 pg/mL, p = 0.0006). No statistical difference was observed in males with PPCS (p = 0.83). Follow-up blood samples were collected at a median of 11.4 months (IQR: 10.3–12.7) after the trauma and following the completion of the non-pharmacological intervention/reference treatment. Figure 3 and Table 4 shows that CGRP levels decreased at follow-up in PPCS participants, with a median change of – 1.3 pg/mL (95% CI – 17.6–0, p = 0.024). The observed change was primarily attributed to females, who showed a median difference of – 2.7 pg/mL (95% CI – 37.4–0, p = 0.028) whereas males did not show any statistical difference (p = 0.54).
Violin plots of serum calcitonin gene-related peptide (CGRP) concentrations in baseline RCT participants with persistent post-concussion symptoms (PPCS) and healthy individuals not participating in the RCT. The median and interquartile range are displayed. Corresponding numbers are presented in Table 3. *p = 0.050. ***p = 0.0006. CGRP Calcitonin gene-related peptide, RCT Randomized controlled trial, PPCS Persistent post-concussion symptoms
Baseline and follow-up serum calcitonin gene-related peptide concentrations in patients with persistent post-concussion symptoms. Baseline blood samples were taken at a median of 3.9 months after the trauma and the follow-up blood samples were taken at a median of 11.4 months after the trauma. Corresponding numbers are provided in Table 4. *p ≤ 0.05. CGRP Calcitonin gene-related peptide, PPCS Persistent post-concussion symptoms
There was no effect of the non-pharmacological intervention on CGRP levels: The median delta CGRP concentration was – 3.5 pg/mL (IQR: – 64.8–0) in the reference treatment (EUC), and 0 pg/mL (IQR: – 46.4–14.9) in the intervention arm (GAIN), and there was no statistical difference (95% CI of median difference: – 15.7–47.3, p = 0.33).
CGRP concentrations ranged from 2 pg/mL to 1000 pg/mL, indicating large interindividual variations in CGRP concentrations (Suppl. Figure 1). However, within each individual, baseline and follow-up concentrations showed a strong correlation (rho = 0.95, p < 0.0001).
Exploratory analyses at baseline
At baseline, high CGRP concentrations were associated with a more favorable outcome in several patient-reported outcome measures. For example, higher serum CGRP correlated weakly with fewer headache days, shorter headache duration, and less headache pain with p-values ranging from 0.038 to 0.050 at baseline (rho ≈ – 0.23) (Fig. 4). However, the Kruskal–Wallis test showed no CGRP concentration difference between headache phenotypes (p = 0.67). Further exploratory analyses (Figs. 5 and 6) showed that higher serum CGRP was correlated with a better physical function, physical health, and less bodily pain on the SF-36 questionnaire (rho = 0.29–0.35, p ≤ 0.0079). Likewise, higher CGRP levels were correlated with a smaller symptom burden as measured by the BDS and WI-8 questionnaires (rho = – 0.29, p = 0.006). However, the only correlation that remained statistically significant after Bonferroni correction was the bodily pain domain on the SF-36 questionnaire (rho = 0.35, p = 0.0008).
An additional observation unrelated to PPCS was a higher median CGRP concentration among healthy males compared with healthy females as shown in Table 3 (201.8 pg/mL vs. 32.1 pg/mL, p = 0.0025), with a median difference of 169.7 pg/mL (95% CI: 14.2–496).
Exploratory analyses at follow-up
The correlations between the change in CGRP concentrations (delta CGRP) and the change in questionnaire scores (delta score) from baseline to follow-up were examined (follow-up minus baseline). Unlike the baseline data, the follow-up questionnaire data and blood samples were obtained at different days, and the median time difference was 27 days (IQR: 9–50). Changes in headache days, duration, current headache pain, and changes in the SF-36 questionnaire scores were not correlated to changes in the CGRP concentration (Suppl. Figures 2 & 3). However, Fig. 7 shows that a reduction in CGRP at follow-up was associated with an improved RPQ and BDS-score, and the latter finding remained significant even after Bonferroni correction (rho = 0.54, p < 0.0001). A post hoc analysis revealed that the significant difference was mainly driven by an improvement in general symptoms such as headache and dizziness as well as cardiopulmonary/autonomic symptoms (Suppl. Table 2).
Change in CGRP (follow-up minus baseline) versus change in patient-reported outcomes (follow-up minus baseline). The sample size was n = 37 for the Headache Impact Test, and n = 50/51 for the remaining questionnaires. Significance level: 0.0013 (Bonferroni corrected). The blue color marks the analysis that survived the Bonferroni correction
Discussion
This study aimed to examine serum CGRP levels in participants with PPCS including PTH 2–6 months after a concussion. A novel finding is that elevated serum CGRP concentrations were observed in PPCS participants compared with healthy individuals. At follow-up, we observed a statistically significant reduction in CGRP levels, which correlated with a reduction in symptom levels. Although elevated CGRP levels have not previously been reported in PPCS, it is a common finding in migraine [31], and the clinical similarities suggest a potential role for CGRP in the pathophysiology of PTH. In fact, a recent study showed that CGRP infusion in patients with PTH could induce migraine-like headache [32]. In our study, > 70% reported headache with migraine-like features, suggesting the possible involvement of CGRP in this cohort as well. Interestingly, treatment with a CGRP antibody (erenumab) reduced the intensity and frequency of headache in a recent open-label study in PTH patients, suggesting its potential as a future treatment for PTH, similar to its use in migraine [33]. However, the evidence is limited, and placebo-controlled RCT studies are needed to draw firm conclusions.
The increased CGRP levels at baseline, and the reduction in CGRP levels at follow-up in PPCS participants were driven by females in this study. Interestingly, this is in accordance with a recent animal study showing increased serum CGRP concentrations after 7 days in female rats, but not in males after an induced head injury [34]. Females have a greater risk of developing PPCS/PTH [35], prompting speculation about a potential link between CGRP and this sex difference, similar to observations in migraine [36]. However, because of the limited sample size of males with PPCS in the present study (n = 19), firm conclusions on CGRP differences between PPCS males and healthy males are not possible due to a power issue, as evidenced by the wide confidence interval (Table 3).
Our findings of higher CGRP concentrations are in contrast with our primary hypothesis predicting lower CGRP concentrations in PPCS patients than those in healthy individuals. Our hypothesis was based on a previous study, which demonstrated lower CGRP plasma concentrations in PTH patients than in healthy controls [14]. The difference could be attributed to the inclusion criteria. In the present study, the mean disease duration was 4 months and only young participants were included compared to a mean disease duration of 49 months and a mean age of 36 in the previous study.
Exploratory analyses – baseline data
Unexpectedly, we observed that higher CGRP levels correlated with fewer headache days, shorter headache duration and reduced current headache pain at baseline (rho ≈ – 0.23). Similarly, elevated CGRP levels correlated with a lower symptom burden, although bodily pain measured by the SF-36 was the only finding that remained statistically significant after Bonferroni correction. The fact that high CGRP concentrations are correlated with a more positive outcome, contradicts findings in migraine [31] and the majority of pain-related studies [37]. However, it could align with the findings in the previously mentioned PTH study [14]. This earlier study included patients refereed to a specialized headache clinic, indicating more severe headaches. Interestingly, their study demonstrated lower CGRP levels in PTH than controls and the same tendency toward an inverse relationship with monthly headache days although it was not statistically significant (rho = – 0.11, p = 0.27). This tendency was shown in a recent study conducted in post-deployment soldiers as well (rho = – 0.12, p = 0.063) [38]. It is thus possible that more severe PTH are associated with lower CGRP levels. An explanation for lower CGRP given in the previous PTH study was that constant headache may result in the depletion of CGRP in trigeminal afferents [14]. This speculation was based on the finding that CGRP tissue levels were depleted in rats following capsaicin injection in the paw skin and sciatic nerve [39]. Whether capsaicin-mediated pain is comparable to the pain in headache is unknown.
However, other explanations can account for our findings as well. CGRP is a neuropeptide with several physiological functions unrelated to headache [13]. In the aforementioned animal study, CGRP inhibition with antibodies in concussed female rats did not alleviate cephalic pain hypersensitivity, raising questions about the role of peripheral CGRP in headache in females [34]. In contrast, there are several studies showing that CGRP inhibition could alleviate symptoms in rodents with migraine-like behavior in both males and females [40]. Furthermore, in a recent study on severe traumatic brain injury in humans, high CGRP concentrations were correlated with a lower risk of mortality, indicating a potential beneficial role for CGRP [41]. Additionally, an animal study showed that CGRP may have a favorable impact on peripheral nerve regeneration [42]. Further studies are needed to establish the role of CGRP in concussion and PTH.
A noteworthy observation, unrelated to PPCS, in our study was that healthy males had higher serum levels of CGRP than healthy females. This align with a recent study in post-deployment soldiers [38], but contrasts with a previous study that showed the opposite in females, particularly among those using oral contraceptives [43]. Studies investigating sex differences in CGRP among healthy individuals are limited, and it was not the primary aim of this study; further research is needed to draw definite conclusions.
Exploratory analyses – follow-up data
At follow-up we showed that a reduction in CGRP levels was correlated with improved symptom levels measured by the BDS questionnaire. The post hoc analysis revealed that the reduction in CGRP correlated especially with an improvement in headache, dizziness and autonomic/cardiopulmonary symptoms (Suppl. Table 2). This suggests that a CGRP reduction could be linked to a positive physiological response. A major limitation in the follow-up data was that the questionnaire data and blood samples were collected at different time points, with a median time difference of 27 days. This could have contributed to the lack of an association between delta CGRP and change in headache days, duration, and pain, particularly when considering the limited response rate in the follow-up headache questionnaire (Suppl. Figure 2). Finally, the RCT intervention did not show any effect on CGRP concentrations at follow-up. This was expected and stated in the preregistered analysis plan since the RPQ-difference (symptom levels) was only 7 points between the intervention arms [15].
In conclusion of our exploratory analyses at baseline and follow-up, we found an association between CGRP levels and patient-reported outcomes measured by questionnaires, which is a rare finding. Future studies should replicate these findings, and additional research is needed to establish the role of CGRP in PPCS/PTH and concussion.
CGRP assay
The CGRP concentrations in this study varied substantially with concentration levels ranging below detectable levels (< 2 pg/mL) to above 1000 pg/mL (Suppl. Figure 1). This wide concentration range is larger than those observed in migraine studies [31]. Apart from differences in inclusion criteria and demographic characteristics (such as sex), variations in assays and methodology can affect CGRP concentrations [44]. It is worth noting that a previous study using a similar methodology and same ELISA kit as our study showed a comparable concentration range, mean, and data distribution in healthy individuals (Suppl. Figure 4) [45].
Strengths and limitations
This study had several strengths. The cohort was well-characterized, the concussion diagnosis was validated using the WHO criteria, and we had a relatively large sample size.
We used an ELISA-kit with no cross-reactivity with calcitonin, a peptide fragment of CGRP (CGRP position 8-37), amylin, and substance P according to the manufacturer. Furthermore, the assay was based on two antibodies for CGRP (sandwich ELISA) indicating its specificity for CGRP. In contrast, most other commercial ELISA kits have not investigated cross-reactivity and are only based on one antibody. Moreover, the QC’s and the calibration curves were constructed in study representative serum matrix, which should minimize the risk of matrix effects. Finally, we checked freeze–thaw stability of CGRP in serum to ensure that the reported concentrations in PPCS participants were accurate.
This study also had limitations. The QC’s systematically produced higher than nominal concentrations (170 pg/mL vs. 125 pg/mL), which indicates a slight overestimation of the concentrations in general. However, more importantly, the QC’s were reproducible with an inter-assay and intra-assay CV ≤ 15%. Furthermore, although the manufacturer reported no cross-reactivity between similar molecules, it cannot be ruled out that cross reactivity exists with a related peptide biomarker. However, this seems unlikely since CGRP was completely depleted in CGRP-free serum which was a pool of samples from both patients and healthy individuals. Since samples from patients and healthy individuals were evenly distributed on each ELISA plate, we do not expect these assay related factors to affect the conclusion of this study.
Preanalytical stability of CGRP is another point of concern. Prolonged storage and lack of protease inhibitors may decrease CGRP concentrations [45], although there is conflicting evidence on this matter [46]. In our study, patient samples underwent one freeze–thaw cycle and had a longer storage duration (up to 8 years), in contrast to the samples from healthy individuals (1 year of storage). Despite these possible limitations, our study still revealed significantly higher CGRP levels in PPCS patients than in healthy individuals. A further limitation of the study was the presence of multiple symptoms in addition to headache among PPCS participants, a consequence of the inclusion criteria requiring an RPQ score of 20 or higher. This diversity of symptoms prevented a clear identification of the exact causes of increased CGRP levels in PPCS participants.
Finally, the healthy individuals consisted of anonymous blood donors, for whom no demographic data were available. Danish blood donors tend to have a higher self-reported health and healthier lifestyle than non-donors, which may introduce a selection bias known as “the healthy donor effect” [47]. Although the mechanisms are poorly understood, lifestyle-related factors, such as higher weight, blood pressure [48], and exercise [49], might increase CGRP-concentrations. Since we lacked data on lifestyle factors in both groups, it cannot be ruled out that the observed differences might be partly explained by variations in lifestyle factors rather than PPCS/PTH. The potential confounding effects of preexisting migraines must also be considered since the headache questionnaire utilized did not allow accurate classification of headaches prior to the concussion [19]. However, 75% of the PPCS participants reported less than 15 headache days a year pre-trauma, and none reported using migraine medications before the concussion (Table 1). Furthermore, since migraine is generally not a contraindication for becoming a blood donor, the group of healthy individuals may also include individuals with migraine, thereby reducing the likelihood that this condition skews our findings. Regardless of potential confounding variables, the observed fivefold increase in median CGRP concentration in females with PPCS, compared with healthy individuals, is substantial. For this difference to be solely attributed to confounding factors, these factors would have to exert a strong influence on CGRP levels.
In conclusion, our data are strongly suggesting a role for CGRP in PTH. Future studies should aim to independently verify whether this is the case, preferably in a population with blood samples available before the head trauma (which can be done in athletes), to clearly establish a causal link between CGRP and concussion/PTH in humans. Furthermore, future studies should investigate whether CGRP targeted therapies are effective in PTH in a placebo-controlled RCT design.
Data availability
Anonymized raw data are not publicly available, but are available for research upon request.
References
Peeters W, Van Den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF et al (2015) Epidemiology of traumatic brain injury in Europe. Acta Neurochir 157(10):1683–1696. https://doi.org/10.1007/s00701-015-2512-7
Silverberg ND, Iverson GL, Cogan A, Dams OCK, Delmonico R, Graf MJP et al (2023) The American congress of rehabilitation medicine diagnostic criteria for mild traumatic brain injury. Arch Phys Med Rehabil 104(8):1343–1355. https://doi.org/10.1016/j.apmr.2023.03.036
Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M et al (2021) Epidemiology of traumatic brain injury in europe: a living systematic review [supplementary data; living update 5, search January 2019]. J Neurotrauma 38(10):1411–1440. https://doi.org/10.1089/neu.2015.4126
Feigin VL, Theadom A, Barker-Collo S, Starkey NJ, McPherson K, Kahan M et al (2013) Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol 12(1):53–64. https://doi.org/10.1016/S1474-4422(12)70262-4
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M et al (2019) Estimating the global incidence of traumatic brain injury. J Neurosurg 130(4):1080–1097. https://doi.org/10.3171/2017.10.jns17352
Cancelliere C, Verville L, Stubbs JL, Yu H, Hincapié CA, Cassidy JD et al (2023) Post-concussion symptoms and disability in adults with mild traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma 40(11–12):1045–1059. https://doi.org/10.1089/neu.2022.0185
Lumba-Brown A, Teramoto M, Bloom OJ, Brody D, Chesnutt J, Clugston JR et al (2020) Concussion guidelines step 2: evidence for subtype classification. Neurosurgery 86(1):2–13. https://doi.org/10.1093/neuros/nyz332
Larsen EL, Ashina H, Iljazi A, Al-Khazali HM, Seem K, Ashina M et al (2019) Acute and preventive pharmacological treatment of post-traumatic headache: a systematic review. J Headache Pain. https://doi.org/10.1186/s10194-019-1051-7
Ashina H, Eigenbrodt AK, Seifert T, Sinclair AJ, Scher AI, Schytz HW et al (2021) Post-traumatic headache attributed to traumatic brain injury: classification, clinical characteristics, and treatment. Lancet Neurol 20(6):460–469. https://doi.org/10.1016/s1474-4422(21)00094-6
Mavroudis I, Ciobica A, Luca AC, Balmus IM (2023) Post-traumatic headache: a review of prevalence, clinical features, risk factors, and treatment strategies. J Clin Med. https://doi.org/10.3390/jcm12134233
Goadsby PJ, Edvinsson L, Ekman R (1990) Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28(2):183–187. https://doi.org/10.1002/ana.410280213
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J (2002) CGRP may play a causative role in migraine. Cephalalgia 22(1):54–61. https://doi.org/10.1046/j.1468-2982.2002.00310.x
Russo AF, Hay DL (2023) CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev 103(2):1565–1644. https://doi.org/10.1152/physrev.00059.2021
Ashina H, Al-Khazali HM, Iljazi A, Ashina S, Jørgensen NR, Amin FM et al (2020) Low plasma levels of calcitonin gene-related peptide in persistent post-traumatic headache attributed to mild traumatic brain injury. Cephalalgia 40(12):1276–1282. https://doi.org/10.1177/0333102420941115
Thastum MM, Rask CU, Næss-Schmidt ET, Tuborgh A, Jensen JS, Svendsen SW et al (2019) Novel interdisciplinary intervention, GAIN, vs. enhanced usual care to reduce high levels of post-concussion symptoms in adolescents and young adults 2–6 months post-injury: A randomised trial. EClinicalMedicine. 17:100214. https://doi.org/10.1016/j.eclinm.2019.11.007
Holm L, Cassidy JD, Carroll LJ, Borg J (2005) Summary of the WHO collaborating centre for neurotrauma task force on mild traumatic brain injury. J Rehabil Med 37(3):137–141. https://doi.org/10.1080/16501970510027321
King NS, Crawford S, Wenden FJ, Moss NE, Wade DT (1995) The Rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 242(9):587–592. https://doi.org/10.1007/bf00868811
Thastum MM, Rask CU, Naess-Schmidt ET, Jensen JS, Frederiksen OV, Tuborgh A et al (2018) Design of an early intervention for persistent post-concussion symptoms in adolescents and young adults: a feasibility study. NeuroRehabilitation 43(2):155–167. https://doi.org/10.3233/nre-172391
Kothari SF, Eggertsen PP, Frederiksen OV, Thastum MM, Svendsen SW, Tuborgh A et al (2022) Characterization of persistent post-traumatic headache and management strategies in adolescents and young adults following mild traumatic brain injury. Sci Rep 12(1):2209. https://doi.org/10.1038/s41598-022-05187-x
Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A et al (2003) A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 12(8):963–974. https://doi.org/10.1023/a:1026119331193
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (2018) Cephalalgia. 38(1):1–211. https://doi.org/10.1177/0333102417738202
Ware JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 30(6):473–83
Budtz-Lilly A, Fink P, Ørnbøl E, Vestergaard M, Moth G, Christensen KS et al (2015) A new questionnaire to identify bodily distress in primary care: The ‘BDS checklist.’ J Psychosom Res 78(6):536–545. https://doi.org/10.1016/j.jpsychores.2015.03.006
Carstensen TBW, Ørnbøl E, Fink P, Pedersen MM, Jørgensen T, Dantoft TM et al (2020) Detection of illness worry in the general population: a specific item on illness rumination improves the Whiteley Index. J Psychosom Res 138:110245. https://doi.org/10.1016/j.jpsychores.2020.110245
Spence M, Moss-Morris R, Chalder T (2005) The Behavioural Responses to Illness Questionnaire (BRIQ): a new predictive measure of medically unexplained symptoms following acute infection. Psychol Med 35(4):583–593. https://doi.org/10.1017/s0033291704003484
Broadbent E, Petrie KJ, Main J, Weinman J (2006) The brief illness perception questionnaire. J Psychosom Res 60(6):631–637. https://doi.org/10.1016/j.jpsychores.2005.10.020
Søgaard HJ, Bech P (2009) Psychometric analysis of common mental disorders – Screening Questionnaire (CMD-SQ) in long-term sickness absence. Scand J Public Health 37(8):855–863. https://doi.org/10.1177/1403494809344653
Eskildsen A, Dalgaard VL, Nielsen KJ, Andersen JH, Zachariae R, Olsen LR et al (2015) Cross-cultural adaptation and validation of the Danish consensus version of the 10-item Perceived Stress Scale. Scand J Work Environ Health 41(5):486–490. https://doi.org/10.5271/sjweh.3510
Frobert Y, Nevers MC, Amadesi S, Volland H, Brune P, Geppetti P et al (1999) A sensitive sandwich enzyme immunoassay for calcitonin gene-related peptide (CGRP): characterization and application. Peptides 20(2):275–284. https://doi.org/10.1016/s0196-9781(98)00172-7
Bioanalytical Method Validation Guidance for Industry - Food and Drug Administration (FDA). https://www.fda.gov/media/70858/download (2018). Accessed 21–11–2023
Kamm K (2022) CGRP and migraine: what have we learned from measuring CGRP in migraine patients so far? Front Neurol 13:930383. https://doi.org/10.3389/fneur.2022.930383
Ashina H, Iljazi A, Al-Khazali HM, Do TP, Eigenbrodt AK, Larsen EL et al (2022) CGRP-induced migraine-like headache in persistent post-traumatic headache attributed to mild traumatic brain injury. J Headache Pain 23(1):135. https://doi.org/10.1186/s10194-022-01499-5
Ashina H, Iljazi A, Al-Khazali HM, Eigenbrodt AK, Larsen EL, Andersen AM et al (2020) Efficacy, tolerability, and safety of erenumab for the preventive treatment of persistent post-traumatic headache attributed to mild traumatic brain injury: an open-label study. J Headache Pain 21(1):62. https://doi.org/10.1186/s10194-020-01136-z
Bree D, Mackenzie K, Stratton J, Levy D (2020) Enhanced post-traumatic headache-like behaviors and diminished contribution of peripheral CGRP in female rats following a mild closed head injury. Cephalalgia 40(7):748–760. https://doi.org/10.1177/0333102420907597
Skandsen T, Stenberg J, Follestad T, Karaliute M, Saksvik SB, Einarsen CE et al (2021) Personal factors associated with postconcussion symptoms 3 months after mild traumatic brain injury. Arch Phys Med Rehabil 102(6):1102–1112. https://doi.org/10.1016/j.apmr.2020.10.106
Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, MaassenVanDenBrink A (2019) Gender aspects of CGRP in migraine. Cephalalgia 39(3):435–444. https://doi.org/10.1177/0333102417739584
Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M (2017) Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain 18(1):34. https://doi.org/10.1186/s10194-017-0741-2
Scher AI, McGinley JS, VanDam LR, Campbell AM, Chai X, Collins B et al (2023) Plasma calcitonin gene-related peptide and nerve growth factor as headache and pain biomarkers in recently deployed soldiers with and without a recent concussion. Headache. https://doi.org/10.1111/head.14635
Donnerer J, Amann R, Schuligoi R, Skofitsch G (1996) Complete recovery by nerve growth factor of neuropeptide content and function in capsaicin-impaired sensory neurons. Brain Res 741(1–2):103–108. https://doi.org/10.1016/s0006-8993(96)00905-5
Wattiez AS, Wang M, Russo AF (2019) CGRP in animal models of migraine. Handb Exp Pharmacol 255:85–107. https://doi.org/10.1007/164_2018_187
Chen LX, Zhang WF, Wang M, Jia PF (2018) Relationship of calcitonin gene-related peptide with disease progression and prognosis of patients with severe traumatic brain injury. Neural Regen Res 13(10):1782–1786. https://doi.org/10.4103/1673-5374.238619
Toth CC, Willis D, Twiss JL, Walsh S, Martinez JA, Liu WQ et al (2009) Locally synthesized calcitonin gene-related Peptide has a critical role in peripheral nerve regeneration. J Neuropathol Exp Neurol 68(3):326–337. https://doi.org/10.1097/NEN.0b013e31819ac71b
Valdemarsson S, Edvinsson L, Hedner P, Ekman R (1990) Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest 50(4):385–388. https://doi.org/10.3109/00365519009091595
Frederiksen SD, Bekker-Nielsen Dunbar M, Snoer AH, Deen M, Edvinsson L (2020) Serotonin and neuropeptides in blood from episodic and chronic migraine and cluster headache patients in case-control and case-crossover settings: a systematic review and meta-analysis. Headache J Head Face Pain. 60(6):1132–64. https://doi.org/10.1111/head.13802
Messlinger K, Vogler B, Kuhn A, Sertel-Nakajima J, Frank F, Broessner G (2021) CGRP measurements in human plasma - a methodological study. Cephalalgia 41(13):1359–1373. https://doi.org/10.1177/03331024211024161
Schifter S (1991) Circulating concentrations of calcitonin gene-related peptide (CGRP) in normal man determined with a new, highly sensitive radioimmunoassay. Peptides 12(2):365–369. https://doi.org/10.1016/0196-9781(91)90027-m
Brodersen T, Rostgaard K, Lau CJ, Juel K, Erikstrup C, Nielsen KR et al (2023) The healthy donor effect and survey participation, becoming a donor and donor career. Transfusion 63(1):143–155. https://doi.org/10.1111/trf.17190
Schifter S, Krusell LR, Sehested J (1991) Normal serum levels of Calcitonin Gene-Related Peptide (CGRP) in Mild to moderate essential hypertension. Am J Hypertens 4(7_Pt_1):565–9. https://doi.org/10.1093/ajh/4.7.565
Tarperi C, Sanchis-Gomar F, Montagnana M, Danese E, Salvagno GL, Gelati M et al (2020) Effects of endurance exercise on serum concentration of calcitonin gene-related peptide (CGRP): a potential link between exercise intensity and headache. Clin Chem Lab Med 58(10):1707–1712. https://doi.org/10.1515/cclm-2019-1337
Transfusionsmedicinske Standarder - Dansk Selskab for Klinisk Immunologi. https://dski.dk/wp-content/uploads/2023/01/tms-5.4-final.pdf (2023). Accessed 21–11–2023
Acknowledgements
We thank Margrethe Kjeldsen, a skilled and experienced bioanalytical technician, for her diligent analyses of all samples. Oana-Veronica Frederiksen, MD, is acknowledged for her notable work in the acquisition of the blood samples from PPCS participants. We express our gratitude to Mille Møller Thastum, neuropsychologist and PhD, for providing data from the original RCT study. We are grateful to Jakob Nebeling Hedegaard, statistician, for valuable feedback on the bootstrap analysis. Finally, we thank Mette Mølby Nielsen, Astrid Nørkjær Frost, and Malene Lundfold Andersen for assisting in recruiting healthy blood donors.
Funding
Open access funding provided by Aarhus Universitet. This work was supported by Helsefonden, A.P. Møller og Hustru Chastine Mc-Kinney Møllers Fond til almene Formaal, Helga og Peter Kornings Fond, Direktør Emil C. Hertz og hustru Inger Hertz’ Fond, the Aarhus County Research Initiative, and Region Midtjyllands Strategiske Forskningsmidler.
Author information
Authors and Affiliations
Contributions
P.P.E. and J.P. conceptualized the study and played major roles in the acquisition of the data. P.P.E. also analyzed and interpreted the data and prepared the original draft. J.P. further contributed to data interpretation and manuscript revision. D.L.H. provided critical advice on CGRP measurements and revised the manuscript. H.W.S., R.K.O., and J.F.N. conceptualized the study, interpreted the data, and contributed to manuscript revision.
Corresponding author
Ethics declarations
Conflicts of interest
H.W.S. has received personal fees from AbbVie, Teva, Lundbeck, Novartis, & Lilly, outside of the submitted work. D.L.H. has received research support from AbbVie, and has acted as an advisor, speaker or consultant for Amgen, Teva and Eli Lilly in the past three years. P.P.E, J.P., R.K.O, and J.F.N. report no conflicts of interests.
Ethics approval and registrations
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Committee of Health Research Ethics of the Central Denmark region (case no. 1-10-72-237-14). All PPCS participants provided written informed consent. Written informed consent was not required from the healthy individuals as it comprised of blood samples from anonymized Danish blood donors all of whom have consented to the use of their anonymized samples for research [50]. The previously published RCT study (GAIN 1.0) was registered on ClinicalTrials.gov (NCT02337101) in January 2015 and published in 2019 [15]. In February 2023, before measuring CGRP, we uploaded and registered the hypotheses and a statistical analysis plan for the primary outcome of this study (NCT05812742).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eggertsen, P.P., Palmfeldt, J., Schytz, H.W. et al. Serum calcitonin gene-related peptide in patients with persistent post-concussion symptoms, including headache: a cohort study. J Neurol 271, 2458–2472 (2024). https://doi.org/10.1007/s00415-024-12181-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12181-y