Abstract
Objective
Nearly 60% of migraine patients treated with monoclonal antibodies (mAbs) targeting the calcitonin gene-related peptide (CGRP) pathway experience a ≥ 50% reduction in monthly migraine days (MMD) at 12 weeks compared to baseline (responders). However, approximately half of the patients not responding to anti-CGRP mAbs ≤ 12 weeks do respond ≤ 24 weeks (late responders). We assessed frequency and characteristics of patients responding to anti-CGRP mAbs only > 24 weeks (ultra-late responders).
Methods
In this multicenter (n = 16), prospective, observational, real-life study, we enrolled all consecutive adults affected by high-frequency episodic migraine (HFEM: ≥ 8 days/month) or chronic migraine (CM), with ≥ 3 prior therapeutic failures, treated with any anti-CGRP mAbs for ≥ 48 weeks. We defined responders patients with a ≥ 50% response rate ≤ 12 weeks, late responders those with a ≥ 50% response rate ≤ 24 weeks, and ultra-late responders those achieving a ≥ 50% response only > 24 weeks.
Results
A total of 572 migraine patients completed ≥ 48 weeks of anti-CGRP mAbs treatment. Responders accounted for 60.5% (346/572), late responders for 15% (86/572), and ultra-late responders for 15.7% (90/572). Among ultra-late responders, 7.3% (42/572) maintained the ≥ 50% response rate across all subsequent time intervals (weeks 28, 32, 36, 40, 44, and 48) and were considered persistent ultra-late responders, while 8.4% (48/572) missed the ≥ 50% response rate at ≥ 1 subsequent time interval and were classified as fluctuating ultra-late responders. Fifty patients (8.7%) did not respond at any time interval ≤ 48 weeks. Ultra-late responders differed from responders for higher BMI (p = 0.033), longer duration of medication overuse (p < 0.001), lower NRS (p = 0.017) and HIT-6 scores (p = 0.002), higher frequency of dopaminergic symptoms (p = 0.002), less common unilateral pain—either alone (p = 0.010) or in combination with UAS (p = 0.023), allodynia (p = 0.043), or UAS and allodynia (p = 0.012)—a higher number of comorbidities (p = 0.012), psychiatric comorbidities (p = 0.010) and a higher proportion of patients with ≥ 1 comorbidity (p = 0.020).
Conclusion
Two-thirds of patients not responding to anti-CGRP mAbs ≤ 24 weeks do respond later, while non-responders ≤ 48 weeks are quite rare (8.7%). These findings suggest to rethink the duration of migraine prophylaxis and the definition of resistant and refractory migraine, currently based on the response after 2–3 months of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a chronic evolutive neurological disorder that is often under-estimated and under-treated [1, 2]. Early and appropriate treatment for migraine is recommended not only to improve patients’ quality of life but also to prevent disease progression and to reduce the risk of medication overuse. Nonetheless, despite more than one-fourth of migraine patients being eligible for prophylaxis [3, 4], only a small proportion of them use preventive medications (2%-12% in Europe, 16.8% in US). Reasons for this include low disease awareness, barriers to migraine diagnosis and treatment, and the suboptimal efficacy and low tolerability of conventional drugs (standard of care (SoC): beta-blockers, anticonvulsants, tricyclics, calcium-channel antagonist) which limit their use to relatively short periods (4–6 months) [5].
The availability of monoclonal antibodies targeting the CGRP pathway (anti-CGRP mAbs)—medications characterized by an unprecedented balance between efficacy and tolerability—has prompted to reconsider the paradigm of migraine prevention, recommending a much longer prophylactic treatment period (12–18 months) [6]. Notably, studies on extended treatments with anti-CGRP mAbs has brought into question the validity of evaluating efficacy at the conventional 3-month interval, typically employed in randomized controlled trials (RCTs). This threshold might be misleading since a considerable proportion (55%) of individuals not responding to anti-CGRP at 12 weeks do achieve a ≥ 50% response within 24 weeks and implies that 1 out of 5 migraine patients is indeed a late responder [7]. Late response to migraine preventative medication has pathophysiological and clinical implications, suggesting that in a large proportion of patients central desensitization may require a longer time, and emphasizing the need for extended treatment duration in these individuals.
The reversal of mechanisms involved in migraine progression and chronicization might be even slower in some patients. To explore this hypothesis, we conducted a multicenter, prospective, real-life study aimed at assessing the presence of ultra-late responders (> 24 weeks) to anti-CGRP mAbs in a large cohort of patients affected by high-frequency episodic migraine (HFEM: 8–14 days/month) or chronic migraine (CM) with multiple prior therapeutic failures.
Methods
This is an ongoing multicenter, cohort, real-life study involving 16 headache centers across 7 Italian regions. It started on December 2018 as part of the I-NEED (Italian NEw migrainE Drugs database) project, which is under the umbrella of the Italian Migraine Registry (I-GRAINE).
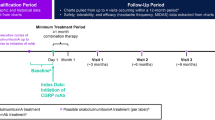
We enrolled all consecutive adult patients affected by HFEM or CM, with ≥ 3 prior therapeutic failures and a MIDAS score > 11—according to the rules of the Italian Medicines Agency—treated with erenumab (70 mg or 140 mg every 28 days), galcanezumab (120 mg following a loading dose of 240 mg every 30 days), or subcutaneous fremanezumab (225 mg every 30 days or 675 mg every 90 days) for ≥ 48 weeks [8]. AntiCGRP mAbs prescription was made based on drug market availability, physician’s choice, or patient’s preference. We excluded patients with fewer than eight migraine days per month, use of onabotulinum toxin A during the previous 12 weeks, prior use of anti-CGRP mAbs or significant cardiovascular or cerebrovascular disorders. No additional preventive medications were started during the observation period.
The study received approval from the IRCCS San Raffaele Roma Institutional Review Board (RP 11/2018) and was mutually recognized by the other local Institutional Review Boards. The study was not preregistered on any study registry site.
After providing written informed consent, all patients were interviewed face-to-face by specifically trained, board-certified headache specialists using a semi-structured, web-based questionnaire that carefully explored sociodemographic and clinical characteristics [9]. In addition to common migraine features (e.g., frequency, pain severity, disability), we also assessed the presence of allodynia, unilateral cranial autonomic symptoms (UAS, defined as ≥ 1 of the following unilateral symptoms during the migraine attack: lacrimation, eye redness, nasal congestion, ptosis, eyelid swelling, miosis or forehead/facial sweating) [10], and presence of dopaminergic symptoms (defined as ≥ 1 of the following symptoms during prodromes, headache stage or postdromes: yawning, somnolence, nausea, vomiting, mood changes, fatigue or diuresis) [11].
We defined responders patients with a ≥ 50% reduction from baseline in monthly migraine days (MMDs) in HFEM or monthly headache days (MHDs) in CM at weeks 9–12, late responders those achieving a ≥ 50% reduction between weeks 13–16 and 21–24, and ultra-late responders those individuals reaching a ≥ 50% reduction only > 24 weeks. The term “headache day” refers to any headache day, including both migraine-like and tension-type like days. We named persistent ultra-late responders those patients who remained ≥ 50% responders at all subsequent time intervals (i.e., weeks 28, 32, 36, 40, 44, and 48) and fluctuating ultra-late responders those not maintaining the 50% response rate at ≥ 1 subsequent time interval. Patients were asked to detail MMDs or MHDs during a 28-day run-in period and across the study using a paper–pencil headache diary.
The primary aim of the study was to assess the presence of ultra-late responders to anti-CGRP mAbs. Additional objectives of the study included profiling ultra-late responders and investigating differences with responders.
Statistical analysis
Convenience sampling was used since previous studies from the same registry demonstrated that the number of patients included in our study was large enough to allows a reliable estimation of response and to compare different subclasses or response. The p-values should be considered as an index of the credibility rather than a test of hypothesis. Categorical variables were represented as numbers and percentages, and their analysis was conducted using the χ2 test or Fisher's exact test when appropriate. Quantitative variables that followed a normal distribution, they were presented as means and standard deviations, otherwise median and interquartile range (IQR) were shown, and nonparametric tests were applied.
To assess changes from baseline in patients at different time points, a paired t-test was utilized. When comparing groups with only one factor and two categories, an independent t-test was applied. If there were more than two categories, ANOVA was used. The proportion of missing data was < 10% for all variables except psychiatric comorbidities and BMI (12% and 11%, respectively), and therefore, a complete case analysis was performed. The study was exploratory in nature, aiming to explore a wide range of potential associations and hypotheses, rather than testing specific pre-defined hypotheses. Therefore, we decided not to apply Bonferroni correction, which is more suitable for confirmatory studies with a limited number of a priori hypotheses, while in exploratory research an overly stringent correction like Bonferroni might stifle the identification of potentially important findings. Sensitivity analyses were carried out excluding one clinical center at a time and examining the impact of the removal on the summary treatment effect. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 28.0 software.
Results
As of March 31, 2023, 1634 patients had received at least 1 dose of anti-CGRP mAbs, while 572 had completed ≥ 48 weeks of treatment, having received at least 12 doses (HFEM/CM:154/418; F/M:432/140; mean age: 48.2 years; erenumab/fremanezumab/galcanezumab: 527/40/5). No patient switched from one anti-CGRP mAb to another, as this is not permitted under current Italian reimbursement rules. Figure 1 depicts the progression of patients along the study. Patients with CM differed from those affected by HFEM in terms of higher body mass index (BMI), monthly analgesic medication use, HIT-6 and MIDAS scores, and a higher frequency of UAS, and treatment failures (Table 1).
Out of the 572 patients who completed ≥ 48 weeks of treatment, 346 (60.5%) showed a ≥ 50% response rate ≤ 12 weeks (responders), while 86 (15%) who did not respond ≤ 12 weeks achieved a ≥ 50% response within 24 weeks (late-responders). Ninety of the 140 subjects non-responding ≤ 24 weeks (15.7%), became treatment responder within 48 weeks (ultra-late responders) (Table 2). Forty-two ultra-late responders (7.3%) maintained the ≥ 50% response rate across all subsequent time intervals (i.e., weeks 28, 32, 36, 40, 44, and 48) and were considered persistent ultra-late responders. Conversely, 48 ultra-late responders (8.4%) missed the ≥ 50% response rate at ≥ 1 subsequent time interval and were classified as fluctuating ultra-late responders. Fifty patients (8.7%) did not respond at any time interval ≤ 48 weeks (Fig. 2). Figure 3 illustrates the reduction from baseline in MMDs/MHDs across 48 treatment weeks in responders, late responders, ultra-late responders, and non-responders. Adverse events, calculated for subject who had received at least one dose of mAbs, occurred in 3.9% (63/1634) of the patients. The most common adverse events were constipation (2.4%), injection site erythema (0.6%), and back pain (0.6%). Serious adverse events were reported by 3 patients (0.18%) who were affected by CM with medication overuse and treated with erenumab. Two of these individuals manifested non-ST segment elevation myocardial infarction, which was considered unrelated to the treatment, while one patient developed a treatment-related paralytic ileus 20 days after receiving the first erenumab 70 mg dose. Only the three patients with serious adverse events discontinued the treatment.
Proportion of patients with a 50% or greater reduction in monthly migraine/headache days following treatment with antiCGRPmAbs in all patients (a), high-frequency episodic migraine (HFEM) (b) and chronic migraine (CM) (c) Light gray bars indicate responders (≤ 12 weeks), white bars non-responders, red bars late-responders (> 12 weeks), blue bars persistent ultra-late responders (> 24 weeks; stable ≥ 50% response across following time intervals), and dashed blue bars fluctuating ultra-late responders (> 24 weeks; instable ≥ 50% response across following time intervals)
A comprehensive analysis comparing responders, late-responders, ultra-late responders, and non-responders for several clinical and demographic parameters, showed significant differences in medication overuse duration, unilateral pain (either alone or associated with UAS, or UAS and allodynia), dopaminergic symptoms, and HIT-6 score and number of comorbidities (Table 2).
Ultra-late responders differed from responders in terms of higher BMI (+ 0.86, 95% CI [0.07;1.66]; p = 0.033), longer medication overuse duration (median 24 vs 6, IQR 3–123 vs 1–15; p < 0.001), lower NRS (− 0.36, 95% CI [− 0.66.0; − 0.06]; p = 0.017), and HIT-6 scores (− 3.16, 95% CI [− 5.16; − 1.15]; p = 0.002). They also exhibited a higher frequency of dopaminergic symptoms (+ 17.6%, 95% CI [0.07;0.27]; p = 0.002), less common unilateral pain either alone − 15.7%, 95% CI [− 26.9; − 4.1]; p = 0.010) or in combination with UAS (− 13.5%, 95% CI [− 23.1; − 2.9]; p = 0.023), allodynia (− 12.1%, 95% CI [− 22.0; − 1.3]; p = 0.043), or UAS and allodynia (− 13.7%, 95% CI [− 21.8;-4.3]; p = 0.012), a higher number of comorbidities (+ 0.32, 95% CI [0.07;0.57]; p = 0.012), psychiatric comorbidities (+ 14.4%, 95%CI [3.1;25.6]; p = 0.010) and a higher proportion of patients with at least 1 comorbidity (+ 14.2%, 95% CI [3.1;24.4]; p = 0.020) (Table 3). Differences between ultra-late responders and responders were primarily driven by CM patients, as detailed in the supplemental Table 1.
Discussion
The present multicenter, prospective, real-life study documents that 15.7% of patients affected by HFEM or CM with multiple prior therapeutic failures are ultra-late responders to anti-CGRP mAbs, achieving a persistent or fluctuating ≥ 50% response rate only after 24 weeks of treatment. This indicates that two-thirds of migraine patients who do not respond at 24 weeks do respond later.
Ultra-late responders do indeed show an early, gradual, progressive reduction in MMDs/MHDs which reaches a ≥ 30% response rate—a clinically meaningful endpoint, at least in CM—between weeks 8 and 24 [12] (Fig. 3). Conversely, non-responders remain persistently below a ≥ 20% response rate throughout the entire 48-week treatment period. Pooling together responders, late responders, and ultra-late responders, the proportion of patients with a ≥ 50% response to anti-CGRP mAbs after 1 year of treatment is 91.3%, while the occurrence of non-responders is quite rare (8.7%).
Why do some migraine patients affected by HFEM or CM respond very late to anti-CGRP mAbs remains unclear. Anti-CGRP mAbs act centripetally, desensitizing trigeminal peripheral nociceptors and subsequently reversing central sensitization. This mechanism becomes clinically evident within 12 weeks, on average, in nearly 60% of patients. However, substantial inter-individual differences may occur. For instance, patients experiencing intense trigemino-vascular activation symptoms (such as unilateral pain, either alone or in association with UAS) appear to be particularly sensitive to anti-CGRP mAbs, exhibiting a notably rapid and high response rate, probably linked to the heightened sensitization of trigeminal nociceptors [9, 13].
By analyzing the migraine phenotype and clinical history of ultra-late responders, we hypothesize that the pathophysiological mechanisms underlying the delayed therapeutic effects of anti-CGRP mAbs could involve reduced trigeminal sensitization, increased central CGRP activity, or both. Reduced sensory activation in ultra-late responders—inferred from their milder and less frequently unilateral pain—could slow down the therapeutic effect of anti-CGRP mAbs. Milder pain in ultra-late responders could also account for the lower HIT-6 score, as this questionnaire is highly sensitive to migraine intensity [14].
On the other hand, the complex clinical picture of ultra-late responders suggests an increased central CGRP activity as indicated by higher BMI values, more numerous comorbidities, psychiatric disorders, dopaminergic symptoms, and longer duration of medication overuse compared to responders [11, 15,16,17]. Therefore, a longer time may be needed to counteract increased central sensitization, delaying the onset of the therapeutic effects of anti-CGRP mAbs.
Ultra-late response to migraine prophylaxis is a relatively unexplored field. Common preventative agents are typically used for short treatment periods (3–6 months) and are often discontinued due to their low tolerability [5]. The introduction of onabotulinum toxin A and anti-CGRP mAbs, medications with a favorable efficacy/tolerability ratio, has paved the way for a new migraine treatment strategy characterized by long-lasting therapies [18, 19]. Prolonged migraine treatment has a solid rationale because migraine progression and chronicization are slowly evolving processes characterized by substantial anatomical, physiological, and biochemical changes in the brain, responsible for increased neuronal excitability [1, 20,21,22]. Increasing migraine frequency is also known to induce brain adaptive changes spreading from central pain networks to areas controlling non-pain behaviors (neurolimbic pain network), thus explaining the occurrence of common comorbidities (psychiatric disorders, fibromyalgia, irritable bowel disease). Consequently, prolonged migraine prophylaxis is needed to reverse progression mechanisms and consolidate the clinical improvement.
Late- and ultra-late responses to migraine preventive medications have meaningful implications for the proper clinical management of the disease. Firstly, they prompt to postpone the assessment of the efficacy of migraine prophylaxis, typically fixed at 12 weeks, to (at least) 24 weeks. Secondly, encourage consideration of extending anti-CGRP mAbs treatment beyond 6 months in patients with a ≥ 30% response at week 12. Thirdly, suggests reconsidering the definition of resistance o refractoriness to migraine prevention, which currently consider treatment response at 2 months for SoC, 3 for anti-CGRP mAbs, and 6 for onabotulinum toxin A [23].
Limitations of the study include the fact that we considered only patients affected by HFEM and CM, thus excluding individuals with low attack frequency, and that most of them were treated with erenumab.
Furthermore, longer follow-up studies are required to mitigate the potential bias resulting from the phenomenon of regression to the mean, which could have influenced our results to some extent [24].
The main strengths are the prospective, multicenter design, the large number of patients consecutively enrolled—representative of northern, central, and southern Italy—and their careful clinical characterization through face-to-face interviews with a shared, web-based semi-structured questionnaire.
In conclusion, this study demonstrates the existence of a significant percentage of patients—otherwise considered non-responders—who actually respond to treatment with anti-CGRP antibodies only after 24 weeks. 91.3% of migraine patients become responders within 48 weeks, with only a small proportion (8.7%) remaining unresponsive. These findings prompt us to rethink the duration of migraine prophylaxis, currently assessed after 2–6 months of treatment, and to reconsider the chronological criteria for defining resistant and refractory migraine. In addition, the presence of late- and ultra-late responders to anti-CGRP mAbs prompts a reconsideration of their reimbursement stopping rules which are presently based on efficacy assessments at 3 or 6 months in various European countries. This time limit appears arbitrary and counterintuitive, as the complexity of mechanisms underpinning migraine progression may necessitate a longer time to observe disease improvement during preventive treatment. The concept of ultra-late-response can contribute to a better comprehension of the mechanisms and the time required for desensitizing the migraine brain.
Change history
02 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00415-024-12287-3
References
Andreou AP, Edvinsson L (2019) Mechanisms of migraine as a chronic evolutive condition. J Headache Pain 20(1):117
Eigenbrodt AK et al (2021) Diagnosis and management of migraine in ten steps. Nat Rev Neurol 17(8):501–514
Katsarava Z, Mania M, Lampl C, Herberhold J, Steiner TJ (2018) Poor medical care for people with migraine in Europe – evidence from the Eurolight study. J Headache Pain 19(1):10
Lipton RB, Nicholson RA, Reed ML, Araujo AB, Jaffe DH, Faries DE, Buse DC, Shapiro RE, Ashina S, Cambron-Mellott MJ, Rowland JC, Pearlman EM (2022) Diagnosis, consultation, treatment, and impact of migraine in the US: Results of the OVERCOME (US) study. Headache 62(2):122–140
Hepp Z, Bloudek LM, Varon SF (2014) Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm 20(1):22–33
Sacco S, Amin FM, Ashina M, Bendtsen L, Deligianni CI, Gil-Gouveia R, Katsarava Z, MaassenVanDenBrink A, Martelletti P, Mitsikostas DD, Ornello R, Reuter U, Sanchez-Del-Rio M, Sinclair AJ, Terwindt G, Uluduz D, Versijpt J, Lampl C (2022) European headache federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention - 2022 update. J Headache Pain 23(1):67. https://doi.org/10.1186/s10194-022-01431-x
Barbanti P, Aurilia C, Egeo G, Torelli P, Proietti S, Cevoli S, Bonassi S (2023) Italian migraine registry study group late response to anti-cgrp monoclonal antibodies in migraine: a multicenter, prospective, observational study. Neurology. https://doi.org/10.1212/WNL.0000000000207292
Gazzetta Ufficiale della Repubblica Italiana. Serie Generale n; 2020. Accessed January 17, 2023. gazzettaufficiale.it/gazzetta/serie_generale/caricaDettaglio? dataPubblicazioneGazzetta=2020–07–21&numeroGazzetta=182.
Barbanti P, Egeo G, Aurilia C et al (2022) Predictors of response to anti-CGRP monoclonal antibodies: a 24-week, multicenter, prospective study on 864 migraine patients. J Headache Pain 23:138. https://doi.org/10.1186/s10194-022-01498-6
Barbanti P, Aurilia C, Dall’Armi V, Egeo G, Fofi L, Bonassi S (2016) The phenotype of migraine with unilateral cranial autonomic symptoms documents increased peripheral and central trigeminal sensitization. a case series of 757 patients. Cephalalgia 36(14):1334–1340
Barbanti P, Aurilia C, Egeo G, Fofi L, Guadagni F, Ferroni P (2020) Dopaminergic symptoms in migraine: a cross-sectional study on 1148 consecutive headache center-based patients. Cephalalgia 40(11):1168–1176
Silberstein S, Tfelt-Hansen P, Dodick DW, Limmroth V, Lipton RB, Pascual J, Wang SJ (2008) Task force of the international headache society clinical trials subcommittee guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 28(5):484–495
Hong JB, Lange KS, Overeem LH, Triller P, Raffaelli B, Reuter U (2023) A scoping review and meta-analysis of anti-CGRP monoclonal antibodies: predicting response. Pharmaceuticals (Basel) 16(7):934. https://doi.org/10.3390/ph16070934
Sauro KM, Rose MS, Becker WJ, Christie SN, Giammarco R, Mackie GF, Eloff AG, Gawel MJ (2010) HIT-6 and MIDAS as measures of headache disability in a headache referral population. Headache 50(3):383–395
Recober A, Goadsby PJ (2010) Calcitonin gene-related peptide: a molecular link between obesity and migraine? Drug News Perspect 23(2):112–117
Mathe AA, Agren H, Lindstrom L, Theodorsson E (1994) Increased concentration of calcitonin gene-related peptide in cerebrospinal fluid of depressed patients a possible trait marker of major depressive disorder. Neurosci Lett 182(2):138–142
D’Antona L, Matharu M (2019) Identifying and managing refractory migraine: barriers and opportunities? J Headache Pain 20(1):89. https://doi.org/10.1186/s10194-019-1040-x
Ray JC, Hutton EJ, Matharu M (2021) OnabotulinumtoxinA in migraine: a review of the literature and factors associated with efficacy. J Clin Med 10(13):2898. https://doi.org/10.3390/jcm10132898
Messina R, Huessler EM, Puledda F, Haghdoost F, Lebedeva ER, Diener HC (2023) Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: A systematic review and network meta-analysis. Cephalalgia 43(3):3331024231152169. https://doi.org/10.1177/03331024231152169
Rattanawong W, Rapoport A, Srikiatkhachorn A (2022) Neurobiology of migraine progression. Neurobiol Pain 9(12):100094. https://doi.org/10.1016/j.ynpai.2022.100094
D’Andrea G, D’Amico D, Bussone G, Bolner A, Aguggia M, Saracco MG, Galloni E, De Riva V, Colavito D, Leon A, Rosteghin V, Perini F (2013) The role of tyrosine metabolism in the pathogenesis of chronic migraine. Cephalalgia 33(11):932–937
Burstein R, Noseda R, Borsook D (2015) Migraine: multiple processes, complex pathophysiology. J Neurosci 35(17):6619–6629
Sacco S, Braschinsky M, Ducros A, Lampl C, Little P, van den Brink AM, Pozo-Rosich P, Reuter U, de la Torre ER, Sanchez Del Rio M, Sinclair AJ, Katsarava Z, Martelletti P (2020) European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine & Headache Alliance (EMHA). J Headache Pain 21(1):76. https://doi.org/10.1186/s10194-020-01130-5
Manack A, Buse DC, Serrano D, Turkel CC, Lipton RB (2011) Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology 76(8):711–718
Funding
This work was partially supported by the Italian Ministry of Health (Institutional Funding Ricerca Corrente) IRCCS San Raffaele Roma and by Fondazione Italiana Cefalee (FICEF).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflicts of interest
Piero Barbanti reports personal compensation for consulting, serving on a scientific advisory board, speaking, research support, collaborated for clinical trials, or other activities with Abbvie, Alder, Allergan, Amgen, Angelini, Assosalute, Bayer, Biohaven, ElectroCore, Eli-Lilly, Fondazione Ricerca e Salute, GSK, Lundbeck, Lusofarmaco, 1MED, MSD, New Penta, Noema Pharma, Novartis, Pfizer, Stx-Med, Teva, Visufarma, Zambon and serves as President with Italian Association of Headache Sufferers. Cinzia Aurilia received travel grants from Eli-Lilly, FB-Health, Lusofarmaco and Teva, honoraria from Novartis, Eli-Lilly and Teva; Gabriella Egeo received travel grants and honoraria from Eli-Lilly, Novartis, New Penta and Ecupharma; Florindo d’Onofrio received travel grant, honoraria as a speaker or for participating in advisory boards from Novartis, Teva, Neopharmed Gentili, Qbgroup srl, K link srl and Eli-Lilly; Paola Torelli received travel grant, honoraria as a speaker, or for participating in advisory boards from Novartis, Teva, Eli Lilly, and Allergan; Cinzia Finocchi received grants and honoraria from Novartis, Eli Lilly, TEVA, AIM group; Sabina Cevoli received honoraria for speaker panels from Teva and Novartis; Maurizio Zucco received travel grants and honoraria from Novartis; Bruno Colombo received travel grants, honoraria for advisory boards, speaker panels or investigation studies from Novartis, Teva, Lilly e Lusofarmaco; Valentina Favoni received honoraria as speaker or for participating in advisory boards from Ely-Lilly, Novartis and Teva; Licia Grazzi received consultancy and advisory fees or honoraria for investigation studies from Allergan, Electrocore LLC, Novartis, Ely-Lilly and Teva; Frediani Fabio received honoraria as speaker or for participating in advisory boards from Novartis, Teva and Eli-Lilly; Claudia Altamura received honoraria for speaker panels from Novartis and Teva; Massimo Filippi is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA); Fabrizio Vernieri received travel grants, honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan, Eli-Lilly, Novartis, and Teva; Stefania Proietti, Marco Aguggia, Davide Bertuzzo, Michele Trimboli, Ilaria Cetta, Monica Laura Bandettini di Poggio, Giulia Fiorentini, Bianca Orlando, Laura Di Clemente, Antonio Salerno, Antonio Carnevale, Micaela Robotti and Stefano Bonassi have no disclosures to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barbanti, P., Aurilia, C., Egeo, G. et al. Ultra-late response (> 24 weeks) to anti-CGRP monoclonal antibodies in migraine: a multicenter, prospective, observational study. J Neurol 271, 2434–2443 (2024). https://doi.org/10.1007/s00415-023-12103-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12103-4