Abstract
Background
Cardiac CT acquired during the acute stroke imaging protocol is an emerging alternative to transthoracic echocardiography (TTE) to screen for sources of cardioembolism. Currently, its diagnostic accuracy to detect patent foramen ovale (PFO) is unclear.
Methods
This was a substudy of Mind the Heart, a prospective cohort in which consecutive adult patients with acute ischemic stroke underwent prospective ECG-gated cardiac CT during the initial stroke imaging protocol. Patients also underwent TTE. We included patients < 60 years who underwent TTE with agitated saline contrast (cTTE) and assessed sensitivity, specificity, negative and positive predictive value of cardiac CT for the detection of PFO using cTTE as the reference standard.
Results
Of 452 patients in Mind the Heart, 92 were younger than 60 years. Of these, 59 (64%) patients underwent both cardiac CT and cTTE and were included. Median age was 54 (IQR 49–57) years and 41/59 (70%) were male. Cardiac CT detected a PFO in 5/59 (8%) patients, 3 of which were confirmed on cTTE. cTTE detected a PFO in 12/59 (20%) patients. Sensitivity and specificity of cardiac CT were 25% (95% CI 5–57%) and 96% (95% CI 85–99%), respectively. Positive and negative predictive values were 59% (95% CI 14–95) and 84% (95% CI 71–92).
Conclusion
Prospective ECG-gated cardiac CT acquired during the acute stroke imaging protocol does not appear to be a suitable screening method for PFO due to its low sensitivity. Our data suggest that if cardiac CT is used as a first-line screening method for cardioembolism, additional echocardiography remains indicated in young patients with cryptogenic stroke, in whom PFO detection would have therapeutic consequences. These results need to be confirmed in larger cohorts.
Similar content being viewed by others
Introduction
Patent foramen ovale (PFO) is present in approximately a quarter of the general population, but is considerably more common in patients with cryptogenic stroke [1,2,3]. PFO closure is indicated in patients younger than 60 years with cryptogenic stroke to reduce stroke recurrence. Although transoesophageal echocardiography (TEE) has been considered the gold standard for diagnosis of PFO, up to one third of stroke patients cannot undergo TEE due to the severity of their stroke, dysphagia, excessive gag reflexes or refusal. Moreover, use of sedation makes the performance of the Valsalva maneuver during TEE more difficult [4, 5]. Previous studies have suggested that transthoracic echocardiography with agitated saline contrast (cTTE) has a similar detection rate for PFO compared to TEE when performed by skilled operators [6, 7]. Therefore, cTTE is most commonly used to screen for PFO [8, 9].
Cardiac CT acquired during the acute stroke imaging protocol is an emerging alternative to TTE to screen for cardioembolism, and a recent study showed that cardiac CT has a higher diagnostic yield than TTE for the detection of high-risk sources of embolism [10]. Currently, the diagnostic accuracy of cardiac CT acquired during the acute stroke imaging protocol to detect PFO is unclear. We, therefore, aimed to assess the sensitivity, specificity, positive predictive value, and negative predictive value of prospective ECG-gated cardiac CT in acute ischemic stroke patients to determine whether it is a suitable screening method for the detection of PFO.
Methods
This was a substudy of the Mind the Heart study, a prospective single-center cohort study in which the diagnostic yield of cardiac CT acquired during the acute stroke work-up was evaluated. Between 2018 and 2020, we included consecutive adult patients with acute ischemic stroke who were potentially eligible for reperfusion therapy (intravenous thrombolysis [IVT] or endovascular treatment [EVT]) at the time of admission (i.e., patients with acute onset neurological symptoms that developed less than 24 h ago). Patients who underwent prospective ECG-gated cardiac CT were triggered to scan during end diastole as part of the initial stroke imaging protocol. Cardiac CT was acquired immediately following non-contrast-enhanced CT of the brain, CT perfusion, and non-gated CT-angiography of the aortic arch, cervical and intracranial arteries. Patients also underwent routine stroke work-up, including TTE. TEE was not routinely performed. Additional details of the study have been published [10]. For the current study, we included all patients who underwent TTE with agitated saline contrast, which was indicated in patients < 60 years in the Mind the Heart study.
Details of the imaging protocol have been published [11]. Presence of PFO was assessed on CT and cTTE according to predefined criteria [11]. On CT, a PFO was defined as a crypt-shaped contrast jet from the left atrium to the right atrium toward the vena cava, or an atrium septum discontinuity [12, 13]. All CT images were systematically assessed by a cardiac radiologist. On cTTE, a PFO was defined as the appearance of microbubbles in the left atrium within 3–6 cardiac beats after opacification of the right atrium [8]. All images were assessed by a cardiologist. During assessment, the cardiac radiologists and cardiologists were blinded to each other’s results. In case of indeterminate results on cardiac CT or cTTE (i.e., due to insufficient image quality), PFO was scored as not being present.
Statistical analysis
Using TTE with agitated saline injection as reference standard, we assessed the sensitivity, specificity, positive predictive value, and negative predictive value for detection of PFO on cardiac CT. Analyses were performed using R software, version 4.0.3 (R foundation for Statistical Computing 2018).
Sensitivity analyses
We determined the sensitivity of cardiac CT when limiting PFO’s to the criteria of the CLOSE study, since these PFO’s are potentially relevant to treat. The criteria of the CLOSE study were: no identifiable cause other than a PFO with an associated atrial septal aneurysm or large interatrial shunt (appearance of more than 30 microbubbles in the left atrium within 3 cardiac cycles after opacification of the right atrium). [14]
We assessed whether the results were consistent when also including patients who were 60 years or older and underwent cTTE.
Results
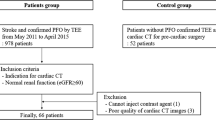
Of 452 patients included in the Mind the Heart study, 92 (20%) patients were younger than 60 years. Of these, 59 (64%) underwent cTTE and were included in the current study (Fig. 1).
Flowchart of patients. TTE transthoracic echocardiography, PFO patent foramen ovale. The reasons for 33 patients not undergoing cTTE were: no echocardiography performed (12 patients, 4 because they died before TTE could be acquired and 8 because TTE in the outpatient setting was too burdensome), echocardiography without contrast performed in another hospital (6 patients), clear other cause of stroke and therefore detection of PFO deemed inconsequential by treating physician (6 patients), failure to acquire IV access (1 patient), and unclear reasons (8 patients)
Median age was 54 (interquartile range [IQR] 49–57) years and 41 (70%) were male (Table 1). In total, 33/59 (56%) patients had cryptogenic stroke and their median Risk of Paradoxical Embolism score was 6 (IQR 6–7). Stroke etiology in the remaining patients was large-artery atherosclerosis in five, cardioembolism in eight, small vessel disease in seven, and other determined in six. There were four indeterminate results on CT, all due to low scan quality, and none on cTTE. Median time difference between cardiac CT and cTTE was 2 (IQR 0–16) days.
Cardiac CT detected a PFO in 5/59 (8%) patients, 3 of which were confirmed on cTTE (Table 2, Figs. 2, 3). cTTE detected a PFO in 12/59 (20%) patients. This resulted in a sensitivity of cardiac CT of 25% (95% confidence interval [95% CI] 5–57%) and specificity of 96% (95% CI 85–99%, Table 3). The positive and negative predictive values were 59% (95% CI 14–95) and 84% (95% CI 71–92), respectively.
Both cardiac CT and cTTE did not detect an atrial septal aneurysm (ASA) in any of the patients with a PFO. One patient with a PFO had a jump-rope interatrial septum detected on echocardiography, though this did not fulfill the criteria for presence of an atrial septal aneurysm (protrusion of > 10 mm beyond the plane of the atrial septum).
Of the 12 patients with a PFO on cTTE, 3 had another identifiable cause of their stroke. Of the remaining nine patients, two did not have a large interatrial shunt according to the CLOSE criteria (< 30 bubbles), meaning that 7 PFO’s fulfilled the criteria of the CLOSE study. Cardiac CT detected a PFO in two of seven of these patients, indicating a similar sensitivity (28%) in this subgroup of patients with a PFO. Results were similar when also including the five patients of 60 years and older who underwent cTTE (supplemental material Tables 1, 2).
Three patients underwent TEE and a PFO was detected on TEE in two of them. Indications for TEE were: assessment if the PFO was eligible for device closure in one, confirmation of PFO detected on cTTE in one, and assessment of the left atrial appendage in the third. No patients underwent transcranial Doppler (TCD) for detection of right–left shunting.
Discussion
In this study, prospective ECG-gated cardiac CT acquired during the acute stroke protocol had a low sensitivity for the detection of PFO in patients with acute ischemic stroke. These data suggest that cardiac CT fails to detect the majority of PFO’s.
The low sensitivity of prospective ECG-gated cardiac CT may be explained by several reasons. First, detection of PFO on CT and TTE is based on different principles. cTTE is used to identify a temporarily right-to-left shunt during the Valsalva maneuver which results in a brief moment of right atrial pressure exceeding the left atrial pressure. In contrast, CT is used to diagnose PFO detecting a left-to-right shunting during rest. The shunt flow through a PFO is determined by differences in pressure between the left and right atrium. Pressure differences between the left atrium and right atrium are the largest during end systole, isovolumetric relaxation, and early diastole [15], while cardiac CT was triggered to scan only during end diastole in our cohort. As such, the false negative findings on CT could be due to equivalent pressures in the left and right atrium at the moment of image acquisition. Moreover, left-to-right shunting may not occur in all patients with a PFO [16,17,18], indicating that these PFO’s may be undetectable on cardiac CT. Furthermore, the applied single-phase prospective ECG-gated cardiac CT acquisition does not allow for dynamic assessment of the heart since only a single phase of the cardiac cycle is scanned, which may further hamper detection of PFO’s. Previous studies compared retrospective ECG-gated cardiac CT, in which the heart is scanned throughout the entire cardiac cycle and images are reconstructed retrospectively, with TEE for the detection of PFO and these studies reported a higher sensitivity and specificity of cardiac CT [15, 19]. However, since more radiation is required for retrospective gated cardiac CT, we decided on prospective ECG gating for our acute stroke imaging protocol.
Although studies have shown that TEE and TCD have a higher diagnostic accuracy than TTE [20], TTE is often the first-line screening method for PFO [8]. Previous studies comparing TEE with TTE and TCD for the detection of PFO reported reasonable sensitivity and specificity for TTE, with the discrepancy between the modalities mainly being driven by false negatives on TTE with very few false positives [21]. Hence, it is likely that if we would have compared prospective gated cardiac CT to TEE or TCD, rather than TTE, the sensitivity of cardiac CT would only have been lower.
A previous meta-analysis of individual patient data of six randomized controlled trials showed that the PFO-Associated Stroke Causal Likelihood (PASCAL) classification performs best in identifying patients who may benefit most from PFO closure [22]. The PASCAL score combines the Risk of Paradoxical Embolism (RoPE) score with additional assessment of high-risk features, either large size of the shunt or presence of atrial septum aneurysm. Although the presence of atrial septum aneurysm can be assessed on prospective ECG-gated cardiac CT, it does not allow for reliable quantification of PFO size. Moreover, cardiac CT detected only 22–73% of atrial septum aneurysms which were diagnosed on echocardiography in previous studies [23, 24]. This provides another argument that if prospective ECG-gated cardiac CT is implemented as a screening method for cardioembolic sources, additional echocardiography is required in patients in whom detection of a PFO has therapeutic consequences.
We were unable to assess the sensitivity of cardiac CT acquired during the acute phase for detection of atrial septal defects (ASD), since none were diagnosed on cTTE. On echocardiography, ASD is diagnosed by detecting a left-to-right shunt. On CT, PFO is differentiated from ASD based on the direction of the left-to-right contrast agent jet, which is toward the vena cava inferior for PFO and perpendicular to the atrial septum for ASD. A previous study showed that retrospective ECG-gated cardiac CT detected all three ASD’s which were diagnosed on TEE [24]. More data are needed to determine whether cardiac CT during the stroke imaging protocol is a suitable screening technique for the detection of ASD.
Prospective gated cardiac CT has a higher diagnostic yield for various high-risk structural sources of embolism than TTE, such as cardiac thrombi [10]. However, our findings suggest that if prospective cardiac CT is implemented in the initial stroke imaging protocol to screen for cardiac structural sources of embolism, echocardiography or TCD will remain essential for PFO detection.
This study has several limitations. First, the sample size is limited, although it is one of the largest studies to date comparing prospective cardiac CT acquired in the acute phase of ischemic stroke to TTE with agitated saline for the detection of PFO. Second, we did not compare CT with TEE or TCD which have a higher diagnostic yield for PFO detection. Third, this was a single-center study, which limits the generalizability of the results. Finally, we cannot exclude that selection bias occurred since 33 patients in our cohort who were younger than 60 years did not undergo cTTE.
Conclusion
Prospective ECG-gated cardiac CT acquired during the acute stroke imaging protocol does not appear to be a suitable screening method for PFO due to its low sensitivity. Our data suggest that if cardiac CT is used as a first-line screening method for cardioembolism, additional echocardiography remains indicated in young patients with cryptogenic stroke, in whom PFO detection would have therapeutic consequences. These results need to be confirmed in larger cohorts.
Data availability
Anonymized data not published within this article will be made available on reasonable request and requires approval by the privacy officer of Amsterdam UMC for GDPR compliance.
References
Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M et al (1988) Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 318:1148–1152
Mazzucco S, Li L, Binney L, Rothwell PM (2018) Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol 17:609–617
Homma S, Sacco RL (2005) Patent foramen ovale and stroke. Circulation 112:1063–1072
Pinto FJ (2005) When and how to diagnose patent foramen ovale. Heart 91:438–440
De Castro S, Papetti F, Di Angelantonio E, Razmovska B, Truscelli G, Tuderti U et al (2010) Feasibility and clinical utility of transesophageal echocardiography in the acute phase of cerebral ischemia. Am J Cardiol 106:1339–1344
Kühl HP, Hoffmann R, Merx MW, Franke A, Klötzsch C, Lepper W et al (1999) Transthoracic echocardiography using second harmonic imaging: diagnostic alternative to transesophageal echocardiography for the detection of atrial right to left shunt in patients with cerebral embolic events. J Am Coll Cardiol 34:1823–1830
Van Camp G, Franken P, Melis P, Cosyns B, Schoors D, Vanoverschelde JL (2000) Comparison of transthoracic echocardiography with second harmonic imaging with transesophageal echocardiography in the detection of right to left shunts. Am J Cardiol 86(1284–1287):a1289
Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM et al (2015) Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American society of echocardiography and society for cardiac angiography and interventions. J Am Soc Echocardiogr 28:910–958
Federatie Medisch Specialisten. Richtijn herseninfarct/hersenbloeding https://richtlijnendatabase.Nl/richtlijn/herseninfarct_en_hersenbloeding/startpagina_herseninfarct_-bloeding.Html. 2017, Accessed 15 Jul 2022
Rinkel LA, Guglielmi V, Beemsterboer CFP, Groeneveld NS, Lobé NHJ, Boekholdt SM et al. Diagnostic yield of ecg-gated cardiac ct in the acute phase of ischemic stroke vs transthoracic echocardiography. Neurology. 2022
Guglielmi V, Rinkel LA, Groeneveld N, e NHJL, Boekholdt SM, Bouma BJ et al (2020) Mind the heart: Electrocardiographygated cardiac computed tomography-angiography in acute ischaemic stroke—rationale and study design. Eur Stroke J 5:441–448
Kamalian S, Kamalian S, Pomerantz SR, Tanpitukpongse TP, Gupta R, Romero JM et al (2013) Role of cardiac and extracranial vascular ct in the evaluation/management of cerebral ischemia and stroke. Emerg Radiol 20:417–428
Pathan F, Hecht H, Narula J, Marwick TH (2018) Roles of transesophageal echocardiography and cardiac computed tomography for evaluation of left atrial thrombus and associated pathology: a review and critical analysis. JACC Cardiovasc Imaging 11:616–627
Mas J-L, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L et al (2017) Patent foramen ovale closure or anticoagulation vs. Antiplatelets after stroke. New Engl J Med. 377:1011–1021
Kim YJ, Hur J, Shim C-Y, Lee H-J, Ha J-W, Choe KO et al (2009) Patent foramen ovale: diagnosis with multidetector ct—comparison with transesophageal echocardiography. Radiology 250:61–67
Sun JP, Stewart WJ, Hanna J, Thomas JD (1996) Diagnosis of patent foramen ovale by contrast versus color doppler by transesophageal echocardiography: relation to atrial size. Am Heart J 131:239–244
Wu CC, Chen WJ, Chen MF, Liau CS, Chu SH, Lee YT (1993) Left-to-right shunt through patent foramen ovale in adult patients with left-sided cardiac lesions: a transesophageal echocardiographic study. Am Heart J 125:1369–1374
Luotolahti M, Saraste M, Hartiala J (1995) Saline contrast and colour doppler transoesophageal echocardiography in detecting a patent foramen ovale and right-to-left shunts in stroke patients. Clin Physiol 15:265–273
Lee S, Kim I-C, Kim YD, Nam HS, Kim SY, Choi SM et al (2021) The role of cardiac ct throughout the full cardiac cycle in diagnosing patent foramen ovale in patients with acute stroke. Eur Radiol. 31:8983–8990
Pristipino C, Sievert H, D’Ascenzo F, LouisMas J, Meier B, Scacciatella P et al (2018) European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur Heart J. 40:3182–3195
Stafford MB, Bagley JE, DiGiacinto D (2019) Comparison of transthoracic echocardiography, transesophageal echocardiography, and transcranial doppler in the detection of patent foramen ovale as the etiology for cryptogenic stroke. J Diagn Med Sonogr 35:127–133
Kent DM, Saver JL, Kasner SE, Nelson J, Carroll JD, Chatellier G et al (2021) Heterogeneity of treatment effects in an analysis of pooled individual patient data from randomized trials of device closure of patent foramen ovale after stroke. JAMA 326:2277–2286
Revel M-P, Faivre J-B, Letourneau T, Henon H, Leys D, Delannoy-Deken V et al (2008) Patent foramen ovale: detection with nongated multidetector ct. Radiology 249:338–345
Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY et al (2009) Cardiac computed tomographic angiography for detection of cardiac sources of embolism in stroke patients. Stroke 40:2073–2078
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CBLMM has received research grants from CVON/Dutch Heart Foundation, European Commission, TWIN Foundation Healthcare Evaluation Netherlands and Stryker (paid to institution). CBLMM, HAM, and YBWEMR are shareholders of Nico.lab, a company that focuses on the use of artificial intelligence for medical image analysis. JMC reports grants from Medtronic, Boehringer Ingelheim, and Bayer outside the submitted work. (paid to institution). The other authors have no financial conflicts of interest.
Ethical approval
This study complies with the Declaration of Helsinki. The medical ethics committee of Amsterdam UMC, location AMC approved the Mind the Heart study (2018_017#C2018275). The study protocol was registered at the Dutch Central Commission for Human Research (CCMO; NL64139.018.18). Patients or their legal representatives provided written informed consent before the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rinkel, L.A., Bouma, B.J., Boekholdt, S.M. et al. Detection of patent foramen ovale in patients with ischemic stroke on prospective ECG-gated cardiac CT compared to transthoracic echocardiography. J Neurol 270, 3537–3542 (2023). https://doi.org/10.1007/s00415-023-11688-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11688-0