Abstract
Introduction
With the approval of natalizumab in Europe in 2006, the Austrian Multiple Sclerosis Therapy Registry (AMSTR) was established. Here, we present data from this registry about effectiveness and safety of natalizumab in patients treated up to 14 years.
Patients/methods
Data retrieved from the AMSTR contained baseline characteristics and biannual documentation of annualised relapse rate (ARR) and Expanded Disability Status Scale (EDSS) score as well as adverse events and reasons for discontinuation on follow-up visits.
Results
A total of 1596 natalizumab patients (71% women, n = 1133) were included in the analysis and the observed treatment duration ranged from 0 to 164 months (13.6 years). The mean ARR was 2.0 (SD = 1.13) at baseline, decreasing to 0.16 after 1 year and 0.01 after 10 years. A total of 325 patients (21.6%) converted to secondary progressive multiple sclerosis (SPMS) during the observational period. Of 1502 patients, 1297 (86.4%) reported no adverse events (AE) during follow-up visits. The most common reported AEs were infections and infusion-related reactions. John Cunningham virus (JCV) seropositivity was the most common specified reason for treatment discontinuation (53.7%, n = 607). There were five confirmed cases of Progressive Multifocal Leukoencephalopathy (PML) with 1 death.
Conclusion
The effectiveness of natalizumab in patients with active relapsing–remitting multiple sclerosis (RRMS) could be confirmed in our real-world cohort even after follow-up of up to 14 years, though after year 10, there were less than 100 remaining patients. A low number of AE were reported in this nationwide registry study, establishing Natalizumab’s favourable safety profile during long-term use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the approval of interferon-beta in Europe in 1995, the therapeutic landscape in multiple sclerosis has evolved significantly [1]. Since then, over a dozen of disease-modifying therapies (DMTs) have been approved, leading to the dilemma of selecting the most appropriate DMT for a given patient. Since pivotal phase 3 trials are usually limited to 2 years, knowledge about long-term safety (and effectiveness) is limited for new DMTs. Furthermore, health authorities ask for real-world effectiveness data to justify the partly enormous costs of DMTs. In Austria, a country with over 13,000 MS patients, the Austrian Multiple Sclerosis Therapy Registry (AMSTR) was therefore established in 2006 [2, 3].
Natalizumab (NTZ), a monoclonal antibody directed against the adhesion molecule α4ß1-integrin (VLA-4), remains one of the most effective immunomodulatory therapies, with progressive multifocal leukoencephalopathy (PML) being the most severe adverse event [4].
The excellent efficacy of NTZ in highly active RRMS has already been demonstrated in pivotal phase III trials, with a 68% reduction in annualized relapse rate (ARR) and a 42% risk reduction for sustained disability progression [5, 6].
The Tysabri Observational Programme (TOP) strengthens these data with a 92.5% reduction in ARR over 10 years in a real-world treatment setting [7]. Furthermore, the TOP 10-year data confirm the favourable long-term safety profile of NTZ. Infections (4.1%) and immune system disorders (1.5%) were the most common side effects, and in particular, the PML rate was 0.9%.
The aim of this paper is therefore to characterize a cohort of NTZ-treated patients from the AMSTR and to analyse follow-up data on effectiveness and safety with a follow up period of up to 14 years.
Methods
In Austria, a dense network of certified MS centres, either hospital-based or office-based, provide high-quality patient care and support, ensuring appropriate use and monitoring of DMT treatment. The Austrian MS Treatment Registry (AMSTR) was established in 2006 to acquire data on effectiveness and safety for approved DMTs in a real-world setting, and thereby to monitor emerging long-term effects and risks. Moreover, data entry into the AMSTR is necessary to comply with reimbursement regulations of the Austrian sick funds. The AMSTR is a secure web-based platform, which requires immediate online documentation during patient visits from treating neurologists in all Austrian MS centres.
The initial entry of baseline data into the AMTR captures: MS onset and duration, relapses in the prior 12 months (ARR), expanded disability status scale (EDSS), MRI activity (3 variables: ≥ 9 T2-hyperintense lesions, ≥ 1 contrast-enhancing lesion [CEL], dynamic of lesion load as compared to a previous scan) and previous DMTs. The follow-up data include: relapses, EDSS, adverse events (AE), change or discontinuation of treatment, anti-JCV antibodies (STRATIFY test), neutralizing anti-NTZ antibodies status and is required to be captured every 3–6 months. In the case of treatment withdrawal, additionally, data regarding the reason for discontinuation are assessed.
All patients were categorized into two groups according to EMA indications for NTZ:
Indication A (escalation strategy): ≥ 1 relapse despite DMT within the last 12 months and ≥ 9 T2 lesions on a recent (not older than 3 months before treatment start) MRI.
Indication B (early intensive strategy): ≥ 2 severe relapses without prior DMT and ≥ 1 gadolinium-enhancing lesion or an increase in T2 lesion burden on a recent MRI compared with a previous scan.
EDSS progression was defined as worsening in the EDSS scale of 0.5 points over two consecutive follow-up visits (equals a median time of 6.2 months). Hence, approximately to the diagnostic criteria of Lorscheider, patients with an EDSS progression of at least 0.5 over at least 6 months without the presence of a relapse in the same time period were therefore rated as secondary-progressive multiple sclerosis (SPMS) [8].
To improve the quality and reliability of the documented data, an independent data monitoring board was established [9]. Since NTZ prescribing and administration is restricted to specialized MS centres, the AMSTR inherently covers NTZ use in Austria. The respective data sets were obtained anonymously and exported from the registry. The study was approved by the Ethics Committee of the Medical University of Vienna (EC number 1448/2020).
Statistics
Descriptive analysis was conducted using IBM SPSS (SPSS Inc. Version 25.0 and 26.0, Chicago, IL, USA). Categorical variables were expressed in frequencies and percentages, continuous variables were described as mean and standard deviation or median and range in accordance with the presence/absence of normal distribution as indicated by Kolmogorov–Smirnov tests.
Univariate correlations were performed by Pearson or Spearman.
Time to on-treatment relapse as well as treatment discontinuation were assessed by survival analyses in terms of Kaplan–Meier curves and log-rank test.
The following cases were censored: no relapse until data retrieval or until drop-out; no drop-out until data retrieval, lost-to-follow-up, drop-out due to relocation, drop-out due to withdrawal of consent, drop-out due to treatment being moved to a non-AMSTR clinic and death.
Group differences were illustrated applying chi2 tests for categorical variables, and independent t-test/Mann–Whitney-U tests for continuous variables with/without normal distribution.
A two-sided p value of 0.05 was considered the level of significance.
Results
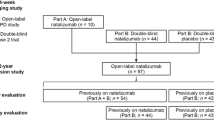
Baseline data in the AMSTR were available for 1602 patients. Six cases were excluded from the baseline analyses due to insufficient data (unavailable date of the first NTZ administration).
Figure 1 gives an overview of the included and excluded patients, including the reasons for exclusion.
Baseline
Table 1 describes the baseline characteristics of this cohort, which includes demographic data, clinical (ARR, EDSS) and paraclinical (MRI) markers of disease activity as well as prior DMT use.
Follow up
The analysis of follow-up visits included 1502 patients who accounted for 20,101 follow-up entries. Patients had a median number of 12 (2–93) follow-up visits and a median time of 4.3 years until last follow-up.
Efficacy
The efficacy of NTZ is illustrated in Fig. 2 with a mean ARR of 0.13 after 2 years and 0.01 after 10 years compared to a mean ARR of 2.0 at baseline.
A total of 432 relapses were documented during the observational period of 0–164 months in 1594 patients. The results indicated a mean survival time (time without relapse) of 112 months (standard error of the mean [SEM] = 2.14) respectively 9.3 years (75th percentile 31 months/2.6 years).
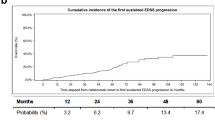
A significantly higher EDSS score (3.26 vs 2.8; p < 0.001) was shown for those with an on-treatment relapse. Baseline ARR values similarly reflected a significant group difference (2.3 vs 1.99 ARR; p < 0.001). There was no difference between on-treatment relapse versus no on-treatment relapse regarding age or disease duration at baseline. A crosstab showed that 840 (52.7%) patients without on-treatment relapses met indication A at baseline and 332 (20.8%) patients met indication B. Among those who experienced an on-treatment relapse, 310 (19.5%) met indication type A and 93 (5.9%) indication type B at baseline (missing data for 1.1%). A χ2-test showed a significant association between baseline indication and on-treatment relapse (χ2 (1) = 4.196; p = 0.041; φ = 0.41). EDSS progression is illustrated in Fig. 3.
N = 325 (21.6%) patients displayed EDSS worsening. For the proportion of patients converting to SPMS per treatment year, see Table 2.
Patients who converted to SPMS within in the first 4 years were significantly older (median 49a vs 43a, p < 0.001), more often male (p < 0.001), had a higher EDSS score at baseline (median EDSS 3 vs 2, p < 0.001), and more often met indication A as compared to indication B criteria(p < 0.001). Noteworthy, data concerning disease duration and ARR did not differ at baseline.
Safety
Out of the 1502 patients included in the analysis of follow-up visits, 1297 (86.4%) patients never indicated any Med DRA (Medical Dictionary for Regulatory Activities) code for adverse events (AE) and n = 205 (13.6%) patients reported at least one Med DRA code. The Med DRA codes reported for most patients were (multiple reports possible): code 1; infections and infestations (n = 51; 24.8%), code 22; general disorders and administration site conditions (n = 48; 23.4%) and code 8; nervous system disorders (n = 45; 21.9%). A detailed overview is given in Table 3.
Cases of PML were documented separately. A total of 5 cases of PML were reported to the registry, of which 1 patient died of PML.
STRATIFY test for anti-JCV-antibodies had been reported for 1,100 (73.2%) patients with 618 (41.1%) patients testing positive at least once. N = 73 (11.8%) of these had a negative test result at baseline and are therefore, likely to have undergone JCV seroconversion. In contrast, 135 (21.8%) already tested positive at baseline and the remaining 410 (66.3%) had no valid data entry for their baseline STRATIFY result.
N = 714 (47.5%) patients were tested for neutralizing anti-NTZ-antibodies at least once, with 28 (1.9%) yielding a positive test result.
Treatment discontinuation
Treatment discontinuation was documented for 1131 patients. Reasons for discontinuing NTZ treatment are summarized in Table 4, with JCV seropositivity (including a few cases with fear of PML instead of JCV seropositivity as specification) being by far the most frequent reason (n = 607, 53.7%).
A total of 714 (63.1%) of cases reported that a follow-up treatment after NTZ discontinuation was planned. Fingolimod was the most frequently reported follow-up therapy (377; 52.8%), followed by glatiramer acetate (63; 8.8%) and intravenous immunoglobulins (IvIg) therapy (37; 5.2%). “Not specified” was indicated for 82 (11.6%) patients.
A Kaplan–Meier curve was created to illustrate drug survival (Fig. 4). Results showed a median survival time of 51 months (4.3 years) with the first percentile at 148 months (12.3 years) and the 75th percentile at 24 months.
NTZ treatment restart
N = 177 patients accounting for 313 data entries were registered to have restarted NTZ treatment. The median duration of treatment pause was 11 months with a range from 0 to 125 months. EDSS scores at restart showed a median of 2.5 and ranged from 0.0 to 7.5. Reoccurrence of relapses was reported as a cause for NTZ restart in 34.5% of cases with a majority (n = 42; 23.7%) indicating that only one relapse occurred. Two relapses were reported in 13 (7.3%) cases, three relapses were reported in 4 (2.3%) cases and 2 (1.1%) cases had five relapses.
Discussion
With up to 14 years of follow-up, the AMSTR provides critical insights into the effectiveness and safety profile of long-term NTZ use in a real-world setting.
Here we provide data confirming the effectiveness of NTZ in a real-world cohort of highly active RRMS patients. The ARR was reduced from 2.0 at baseline to 0.16 (92% reduction of ARR compared to baseline) in the first year of treatment and further decreased to 0.01 (> 99% reduction of ARR compared to baseline) after 10 years.
Moreover, patients remained relapse-free for a mean of 9.3 years, and the proportion of no on treatment relapse was significantly (p = 0.041) higher for those with indication type B (early intensive strategy) reinforcing the meaningfulness of an early active treatment strategy[10]. This is consistent with real-world data available so far and underlines the established use of NTZ as first-line therapy for highly active RRMS patients [7, 11,12,13].
Conversion to secondary progressive MS (SPMS) was observed in 21.6% with a median age of 43 years, representing a typical age of conversion to SPMS [8]. Secondly, with a median disease duration of 14 years, patients were showing a relatively low conversion rate with a relatively late time point of conversion compared to natural history data, especially when considering the fact that this cohort consists of patients with highly active RRMS [14]. Indeed, according to natural history data, a conversion rate to SPMS of around 50% would be expected within 19 years in a cohort with mild to highly active disease courses [15, 16].
Similar to the effectiveness data, our long-term safety results are also in line with available real-world data. The vast majority of the patients (86.4%) did not report any AE. The most common reported AEs were (i) infections and infestations, (ii) general disorders and administration site conditions, and (iii) nervous system disorders.
This is consistent with the findings in the pivotal phase III studies (AFFIRM, SENTINEL), where a slightly increased incidence of urinary tract infections and respiratory infections was observed [5, 6].
In the AMSTR a total of 5 PML cases among 1596 patients (5 confirmed PML cases with 1 death), were captured. Compared to other large surveillance programs (TOP, TYGRIS, STRATA, STRATIFY-2, see Table 5), our data revealed comparable AE rates including PML cases [7, 11,12,13].
Hereafter, Table 5 provides a comparison of the different observation programs with the AMSTR data [7, 11,12,13].
Certain differences could be explained by different sample sizes and differences in data reporting and documentation, i.e., the AMSTR represents a nationwide registry, covering most patients treated with NTZ in Austria, while TOP is an open-label, multinational observational study. Furthermore, it could be due to the smaller sample size in comparison with the international registry studies (see Table 5).
All in all, NTZ represents a clear first-line therapy in the treatment of (highly) active RRMS with an excellent safety profile. The only limiting factor is seroconversion of JCV, especially after 2 or more years of therapy.
The main potential limitations of this registry-based study are: (i) adverse events may not have been reported to the registry (reporting bias), (ii) the number of patients decreased significantly over years on treatment (attrition bias), given a caseload of less than 100 after year 10. Hence, we must emphasize that this impressing efficacy data need to be interpretated with caution as the relatively high drop-out rates in our study may lead to a selection bias towards stable disease patients continuing the study.
Conclusion
In line with real world data available so far, we confirm in a nationwide Austrian registry study the high effectiveness as well as the long-term safety of NTZ in highly active RRMS patients. In particular, no new safety issues including PML numbers occurred, which is in line with international surveillance programmes [7, 11,12,13]. Our data, therefore, support the use of NTZ as a first line therapy in active RRMS patients due to its excellent benefit-risk-profile, with JCV-seroconversion being the only major limitation in the long-term use of NTZ.
Data availability
Anonymized data will be shared by reasonable request from any qualified investigator.
Change history
05 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00415-023-11784-1
References
Tintore M, Vidal-Jordana A, Sastre-Garriga J (2019) Treatment of multiple sclerosis — success from bench to bedside. Nat Rev Neurol 15:53–58
Guger M, Enzinger C, Leutmezer F, Kraus J, Kalcher S, Kvas E et al (2018) Real-life clinical use of natalizumab and fingolimod in Austria. Acta Neurol Scand 137(2):181–187
Salhofer-Polanyi S, Cetin H, Leutmezer F, Baumgartner A, Blechinger S, Dal-Bianco A et al (2017) Epidemiology of multiple sclerosis in Austria. Neuroepidemiology 49(1–2):40–44
Kappos L, Bates D, Hartung HP, Havrdova E, Miller D, Polman CH et al (2007) Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol 6:431–441
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354(9):899–910
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue E-W et al (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354(9):911–923
Butzkueven H, Kappos L, Wiendl H, Trojano M, Spelman T, Spelman T et al (2020) Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry 91(6):660–668
Lorscheider J, Buzzard K, Jokubaitis V, Spelman T, Havrdova E, Horakova D et al (2016) Defining secondary progressive multiple sclerosis. Brain 139(9):2395–2405
Guger M, Enzinger C, Leutmezer F, Di Pauli F, Kraus J, Kalcher S et al (2021) Long-term outcome and predictors of long-term disease activity in natalizumab-treated patients with multiple sclerosis: real life data from the Austrian MS Treatment Registry. J Neurol 268(11):4303–4310
He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB et al (2020) Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 19(4):307–316
O’Connor P, Goodman A, Kappos L, Lublin F, Polman C, Rudick RA et al (2014) Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS study. Neurology 83(1):78–86
Foley J, Carrillo-Infante C, Smith J, Evans K, Ho PR, Lee L et al (2020) The 5-year Tysabri global observational program in safety (TYGRIS) study confirms the long-term safety profile of natalizumab treatment in multiple sclerosis. Mult Scler Relat Disord 39:101863
Campagnolo DI, Ho P-R, Patel R, Chang I, Subramanyam M, Koendgen H et al (2016) Four-year longitudinal index stability data from STRATIFY-2 support the clinical utility of index for risk stratification of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology 87(2):e25
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ et al (2014) Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 83:278–286
Tremlett H, Zhao Y, Devonshire V (2008) Natural history of secondary-progressive multiple sclerosis. Mult Scler 14(3):314–324
Confavreux C, Vukusic S (2006) Natural history of multiple sclerosis: a unifying concept. Brain 129(3):606–616
Acknowledgements
The Steering Group would like to take this opportunity to thank all Austrian MS centres for entering data into the registry and all patients for their informed written consent.
Funding
Open access funding provided by Medical University of Vienna. The Austrian MS Treatment Registry is supported by unrestricted grants of Biogen Austria, Novartis Pharma Austria, and Genzyme Austria.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Patrick Altmann has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Merck, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting from Biogen. He received a research grant from Quanterix International and was awarded a sponsorship from Biogen, Merck, Sanofi-Genzyme, Roche, and Teva to programme a smartphone application for people with MS. Thomas Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Biogen, Bionorica, Celgene/BMS, GSK, Janssen-Cilag, MedDay, Merck, Novartis, Roche, Sanofi Aventis/Genzyme, TG Therapeutics and TEVA. His institution has received financial support in the last 2 years by unrestricted research grants (Biogen, Celgene/BMS, Novartis, Roche, Sanofi Aventis/Genzyme, TEVA) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Roche, Sanofi Aventis/Genzyme, TEVA. Gabriel Bsteh has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Novartis, Roche, Sanofi-Genzyme and Teva. He has received research grants from Celgene/BMS and Novartis. Sarinah Dekany declares no conflict of interest with respect to the study and data presented in this paper. Christian Enzinger received funding for travel and speaker honoraria from Biogen, Bayer, Celgene, Merck, Novartis, Roche, Shire, Genzyme and Teva Pharmaceutical Industries Ltd./sanofi-aventis; research support from Merck Serono, Biogen, and Teva Pharmaceutical Industries Ltd./sanofi-aventis; serving on scientific advisory boards for Bayer, Biogen, Celgene, Merck, Novartis, Roche and Teva Pharmaceutical Industries Ltd./sanofi-aventis. Michael Guger received support and honoraria for research, consultation, lectures and education from Almirall, Bayer, Biogen, Celgene, Genzyme, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi Aventis, Shire, and TEVA ratiopharm. Jörg Kraus received consulting and/or research funding and/or educational support from Almirall, Bayer, Biogen, Celgene/ Bristol Myers Squibb, MedDay, Medtronic, Merck, Novartis, Roche, Sanofi-Aventis, Shire, and TEVA ratiopharm. Barbara Kornek has received honoraria for lecturing or consulting from Biogen, BMS-Celgene, Merck, Novartis, Johnson&Johnson, Sanofi-Genzyme, Roche, and Teva. Fritz Leutmezer has participated in meetings sponsored by, received speaker honoraria or travel funding or unrestricted scientific grants from Actelion, Biogen, Celgene, Med Day, Merck, Novartis, Roche, Sanofi-Genzyme, Schering, and Teva. Tobias Monschein has participated in meetings sponsored by or received travel funding from Biogen, Celgene, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. Markus Ponleitner has participated in meetings sponsored by or received travel funding from Amicus, Merck, Novartis and Sanofi-Genzyme. Paulus Stefan Rommer received honoraria for lectures or consultancy from AbbVie, Alexion, Allmiral, Biogen, Daiichi-Sankyo, Merck, Novartis, Roche, Sandoz, Sanofi Genzyme, Teva. He received research grants from Amicus, Biogen, Merck, and Roche. Franziska Di Pauli has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Almirall, Bayer, Biogen, Celgene, Janssen, Merck, Novartis, Sanofi-Genzyme, Roche, and Teva. Her institution has received research grants from Roche. Tobias Zrzavy has participated in meetings sponsored by or received travel funding from Biogen, Celgene, Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Ethical standard
The study was approved by the Ethics Committee of the Medical University of Vienna (EC number 1448/2020).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monschein, T., Dekany, S., Zrzavy, T. et al. Real-world use of natalizumab in Austria: data from the Austrian Multiple Sclerosis Treatment Registry (AMSTR). J Neurol 270, 3779–3786 (2023). https://doi.org/10.1007/s00415-023-11686-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11686-2