Abstract
Background
Cognitive impairment in people with MS (PwMS) has primarily been investigated using conventional imaging markers or fluid biomarkers of neurodegeneration separately. However, the single use of these markers do only partially explain the large heterogeneity found in PwMS.
Objective
To investigate the use of multimodal (bio)markers: i.e., serum and cerebrospinal fluid (CSF) levels of neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) and conventional imaging markers in predicting cognitive functioning in PwMS.
Methods
Eighty-two PwMS (56 females, disease duration = 14 ± 9 years) underwent neuropsychological and neurological examination, structural magnetic resonance imaging, blood sampling and lumbar puncture. PwMS were classified as cognitively impaired (CI) if scoring ≥ 1.5SD below normative scores on ≥ 20% of test scores. Otherwise, PwMS were defined as cognitively preserved (CP). Association between fluid and imaging (bio)markers were investigated, as well as binary logistics regression to predict cognitive status. Finally, a multimodal marker was calculated using statistically important predictors of cognitive status.
Results
Only higher NfL levels (in serum and CSF) correlated with worse processing speed (r = − 0.286, p = 0.012 and r = − 0.364, p = 0.007, respectively). sNfL added unique variance in the prediction of cognitive status on top of grey matter volume (NGMV), p = 0.002). A multimodal marker of NGMV and sNfL yielded most promising results in predicting cognitive status (sensitivity = 85%, specificity = 58%).
Conclusion
Fluid and imaging (bio)markers reflect different aspects of neurodegeneration and cannot be used interchangeably as markers for cognitive functioning in PwMS. The use of a multimodal marker, i.e., the combination of grey matter volume and sNfL, seems most promising for detecting cognitive deficits in MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment is one of the most disabling symptoms of multiple sclerosis (MS), significantly hampering day-to-day functioning [1]. In an effort to monitor cognitive functioning in MS, understanding its underlying neurobiological correlates is of utmost importance. To date, most studies investigated magnetic resonance imaging (MRI) characteristics in relation to cognitive performance, which has taught us that cognitive impairment is associated with neurodegeneration such as cortical and deep grey matter atrophy [2, 3], as well as with functional impairment of neuronal networks [4]. However, the clinical implementation of these prognostic biomarkers is limited, as these markers cannot fully account for the large heterogeneity found between people with MS (PwMS) [5]. The complex pathology of MS, including inflammation, demyelination and neurodegeneration warrants a multimodal biomarker linking both molecular and imaging biomarkers [6].
Recent studies focused on the combination of neurofilament light chain levels in serum (sNfL) and conventional imaging markers (e.g., lesion load and grey matter volume) for predicting cognitive functioning in PwMS [7, 8]. NfL reflects the major intermediate cytoskeletal protein of axons and is considered to be a marker for neuro-axonal damage [9]. Indeed, increased levels of NfL in the serum and cerebrospinal fluid (CSF) of PwMS have been related to cognitive impairment [10] and decreased performance on multiple cognitive domains [7, 11], in various disease stages [8, 12], showing promising predictive value over time [10]. Another potentially interesting biomarker for the assessment of neurodegeneration in MS is glial fibrillary acidic protein (GFAP), the intermediate cytoskeletal protein of astrocytes [13, 14]. Both serum and CSF levels of GFAP have been shown to relate to disease type (i.e., increased levels of GFAP in progressive PwMS [15]) and disease severity (i.e., increased GFAP levels were associated with higher physical disability and longer disease durations [13, 16]). However, the association between GFAP and cognitive functioning in MS has yet to be established.
The aim of current study was to compare, confirm and combine (bio)markers of neurodegeneration (i.e., serum and CSF levels of both NfL and GFAP and conventional imaging markers) for its role in cognition in a clinical sample of PwMS that visited our outpatient clinic because of perceived cognitive complaints.
Materials and methods
Study population
In total, 129 PwMS that visited the Second Opinion Multiple Sclerosis and Cognition (SOMSCOG) outpatient clinic of the MS Center Amsterdam between February 2017 and November 2020 (82 females, mean age: 48 ± 11 years) were included in this cross-sectional study. Individuals were referred to our SOMSCOG outpatient clinic because of cognitive complaints by their general physician or neurologist, and underwent an extensive diagnostic workup for cognitive impairment, including neuropsychological and neurological examination, MRI, blood sampling and lumbar puncture (all administered on the same day). PwMS were included if they gave written informed consent and had a clinically definite diagnosis of MS according to the McDonald MS criteria (2017–revised) [17] or clinically isolated syndrome. Additionally, PwMS were only included if they underwent a performance validity test (Amsterdam Short-Term Memory (ASTM) test [18, 19]) and had reached a sufficient score on this test, resulting in the exclusion of 35 PwMS. After applying the in- and exclusion criteria, a total of 82 PwMS remained eligible for data-analysis (Fig. 1). This is the second paper including data of this cohort, the first paper focused on performance validity [19].
An overview of the included PwMS after applying the in- and exclusion criteria. APwMS were only included if they underwent a performance validity test (Amsterdam Short-Term Memory test) and had reached a sufficient score on this test. PwMS People with MS, SOMSCOG Second Opinion Multiple Sclerosis and Cognition
Demographics and clinical functioning
The demographic characteristics included age, sex and level of education (coded according the Verhage classification, a Dutch classification system for education [20]). Information on MS type, disease duration, and disease-modifying therapy (DMT; yes/no and if yes, first-line or second-line DMT) was collected from the medical charts. The level of physical disability was assessed by a certified examiner using the Expanded Disability Status Scale (EDSS) [21].
Neuropsychological examination
Cognitive functioning was measured using an Dutch adaptation of the MACFIMS [22], consisting of the following five (sub)-domains: processing speed (Symbol Digit Modalities Test [23] and Stroop Color-Word Test cards I and II [24]), verbal memory (Dutch version of the California Verbal Learning Test version 2 [25]), visuospatial memory (Brief Visuospatial Memory Test—Revised [26]), executive function (EF)-verbal fluency (Controlled Oral Word Association Test [27]) and EF-inhibition (Stroop Color-Word Test Interference score [24]). The neuropsychological examination was administered by a certified clinical neuropsychologist at the department of Medical Psychology of the hospital.
Cognitive test scores were corrected for age, sex and educational level (if predictor was below α = 0.1 in regression analysis [28]), and transformed into five domain-specific Z-scores, based on a normative sample of Dutch healthy controls (N = 407). PwMS were classified as cognitively impaired (CI) if ≥ 20% of the cognitive test scores were ≥ 1.5SD below normative scores (corresponding to ≥ 3 out of 11 test scores), or otherwise as cognitively preserved (CP) [29].
Since multiple psychological factors are known for its impact on cognition, henceforth referred to as patient-reported outcome measures (PROMS), current study protocol included: self-perceived cognitive problems (using the MS Neuropsychological Questionnaire–Patient version, MSNQ-P [30]), symptoms of anxiety and depression (using the Hospital Anxiety and Depression Scale, HADS [31]), levels of fatigue (using the Checklist Individual Strength-20 Revised, CIS20-R [32]), and sleep-related problems (using the Athens Insomnia Scale, AIS [33]). For all aforementioned questionnaires, higher scores indicate more symptoms.
Imaging markers
Seventy-eight PwMS (~ 91%) underwent MR scanning on a 3-Tesla whole-body scanner (General Electric Signa-HDxt, Milwaukee, WI, USA), with an 8-channel head coil (see the Supplemental Methods for the detailed MRI protocol). White matter lesions on FLAIR images were segmented after which lesions on T1-weighted images were filled using an automated lesion-filling technique (LEAP) [34]. The SIENAX pipeline was used to obtain estimates of global white matter and grey matter volumes. FIRST was then applied for the automatic segmentation and calculation of bilateral hippocampus and thalamus volumes, areas known to be associated with cognitive decline in MS. All volumes were corrected for head size using the V-scaling factor obtained by SIENAX.
Fluid biomarkers
Blood samples were collected in serum tubes through venipuncture for 78 PwMS (~ 95%), whereas CSF samples were obtained by lumbar puncture for 54 PwMS (~ 66%; see Supplemental Methods) [35]. Serum and CSF NfL and GFAP levels were quantified in parallel on Single Molecule Array (Simoa) HD-1 analyzers (Quanterix) using the Simoa NF-light Advantage Kit (Quanterix) and the Simoa GFAP Discovery Kit (Quanterix) [36]. Paired CSF and serum samples per PwMS were analyzed within one run. The average intra-assay coefficient of variation (CV) of sample duplicates was 5.3 ± 4.1% for serum GFAP (sGFAP) and 4.1 ± 3.7% for CSF GFAP (cGFAP); NfL measurements were performed in singlicates. One serum NfL (sNfL) measure failed due to a debris error (total N sNfL = 77). For sGFAP, two samples were measured in singlicate and for cGFAP, one sample was measured in singlicate.
Statistical analysis
Normality of variables was explored using the Shapiro–Wilk test and histogram inspection of the residuals. In case of non-normally distributed data, logarithmic (for NLV, sNfL and CSF NfL (cNfL)) or square root transformations (for disease duration, sGFAP and cGFAP) were applied. Fluid and imaging (bio)markers were corrected, regression based, for sex and age (if p value of demographic variable was smaller than α = 0.1 in the model). The following correction formulas were applied:
To investigate differences between CP and CI, independent samples t-tests were applied (for age, disease duration, PROMS, corrected fluid and imaging (bio)markers), or a Mann–Whitney U test for EDSS. Chi-square tests were used to investigate CP vs. CI differences in sex, educational level, type of MS, use of DMT and type of DMT (first-line vs. second-line). Pearson correlations were used to investigate the association (1) between corrected fluid biomarkers and cognitive domains, (2) between corrected imaging markers and cognitive domains and (3) between corrected fluid and imaging (bio)markers. Outcomes were Bonferroni corrected: the α-level of 0.05 was divided by the number of fluid (p < 0.0125) or imaging (bio)markers (p < 0.01).
Two binary logistic regression models (using either serum or CSF markers) with forward selection were run to identify the predictors of cognitive status. The choice of running two separate regressions was made as a significant part of the sample did not receive the lumbar puncture. To reduce the number of variables in the prediction models, the imaging markers were only inserted as predictor if significant group differences were present.
In a post-hoc analysis, the predictive value of the predictors alone was explored, while a weighted composite score of the significant predictors was calculated using the standardized betas. The composite score was further evaluated by drawing receiver operator characteristic curves and calculating the areas under the curves (AUCs) to determine diagnostic accuracy. Interpretation of the AUC was as follows: an AUC of 0.60 and 0.70 could be considered ‘poor’, an AUC of 0.70 to 0.80 could be considered ‘acceptable’ or ‘fair’, an AUC of 0.80 to 0.90 could be considered ‘good’ and an AUC above 0.9 could be considered ‘excellent’ [37]. Significance level was set at α-level < 0.05 and the statistical analyses were performed in SPSS 28.0 (IBM, Armonk, NY, USA).
Results
Study population
The final sample consisted of 82 PwMS (56 females, mean age = 47 ± 9 years, mean disease duration = 14 ± 9 years). Information on demographics, disease related variables, PROMS and imaging and fluid biomarkers can be found in Table 1. In 75% of the PwMS, the MSNQ-P was above the threshold of 27 [38], indicating the presence of self-perceived cognitive problems at the time of the visit. Performance on individual cognitive tests is included in Supplementary Table 2.
Differences between cognitive groups
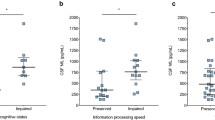
An overview of the differences between groups can be found in Table 1 (in Supplementary Table 3 group differences with only complete data is included). Compared to CP PwMS (N = 36), the group of CI PwMS (N = 46) had worse physical disability (p = 0.012) and consisted of more men (16.7% versus 43.5%; p = 0.010). PROMS results were similar for both groups. CI PwMS had lower NGMV (p = 0.002, d = 0.741, 95% confidence interval (95% CI) [0.278:1.199]), lower thalamic volume (p = 0.002, d = 0.727, 95% CI [0.265:1.185]) and higher NLV (p = 0.010, d = − 0.603, 95% CI [− 1.056: − 0.146]), compared to CP PwMS. As depicted in Fig. 2, increased levels of sNfL and sGFAP were found in CI compared to CP PwMS (p = 0.010, d = − 0.605, 95% CI [− 1.064: − 0.141]; p = 0.035, d = − 0.492, 95% CI [− 0.947: − 0.035], respectively). No differences were found for cNfL and cGFAP. In a final step, a sensitivity analysis was performed. By only including PwMS who have CSF measures available (N = 54), it was checked whether differences between CP and CI PwMS regarding sNfL and sGFAP were still present as confirmation. Although levels of sGFAP were similar between cognitive groups (p = 0.088), levels of sNfL were still increased in CI PwMS (mean sNfL = 12.95 ± 7.75 pg/ml) compared to CP PwMS (mean sNfL = 11.03 ± 10.95 pg/ml; p = 0.043).
Differences in fluid biomarkers between cognitively preserved (CP) and cognitively impaired (CI) PwMS. Results indicate that sNfL and sGFAP are increased in CI PwMS, compared to CP PwMS (*p < 0.05). For illustrative purposes, the raw (non-transformed, not corrected) values of fluid biomarkers are shown. PwMS people with MS, CP cognitively preserved, CI cognitively impaired, sNfL serum neurofilament light (NfL), sGFAP serum glial fibrillary acidic protein (GFAP), cNfL CSF NfL, cGFAP CSF GFAP
Associations between fluid and imaging (bio)markers and cognitive functioning
Fluid biomarkers and cognitive domains
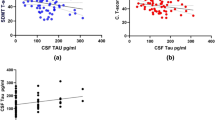
As depicted in Fig. 3, reduced processing speed was associated with increased levels of sNfL (r = − 0.286, p = 0.012) and cNfL (r = − 0.364, p = 0.007).
An overview of the correlations between fluid biomarkers, imaging markers and cognitive domains. The correlation coefficient is displayed inside the blocks. Only significant correlations are shown in color (after correction for multiple comparisons; in italic if borderline significant). Correlations between cognitive domains and a post-hoc calculated composite score (a combination of significant predictors (sNfL and NGMV)) is depicted on the bottom row. sNfL serum neurofilament light (NfL), sGFAP serum glial fibrillary acidic protein (GFAP), cNfL CSF NfL, cGFAP CSF GFAP, NGMV normalized grey matter volume, NWMV normalized white matter volume, NLV normalized lesion volume, EF executive function
Imaging markers and cognitive domains
Reduced processing speed was associated with lower NGMV, thalamic and hippocampal volume (range of coefficients: 0.371–0.422), and increased NLV (r = − 0.376). Reduced verbal and visuospatial memory were associated with lower NGMV and thalamic volume, with only an association with increased NLV for visuospatial memory. Correlation coefficients are included in Fig. 3.
Fluid and imaging (bio)markers
Increased levels of cNfL were associated with reduced thalamic volume (r = − 0.389, p = 0.004) and borderline increased NLV (r = 0.345, p = 0.011, Fig. 3). Finally, increased levels of cGFAP were associated with reduced hippocampal volume (r = − 0.347, p = 0.010), although this finding was borderline significant.
No other correlations between fluid and imaging (bio)markers and cognitive functioning survived correction for multiple comparisons (Fig. 3).
Prediction of cognitive status
In the first logistic regression model, imaging markers (i.e., NGMV, NLV and thalami), sNfL and sGFAP were included as predictors of cognitive status (N = 73). Only NGMV and sNfL were able to predict cognitive status (Table 2). When added to the model, sNfL significantly improved the prediction of cognitive status compared to NGMV alone (sensitivity increased from 70 to 77.5%, whereas the specificity remained 60.6%; p = 0.025). The final model, including NGMV and sNfL, resulted in a sensitivity of 77.5%, a specificity of 60.6%, a positive predictive value (PPV) of 70.5%, a negative predictive value (NPV) of 69.0% and an accuracy of 69.9%. In a post-hoc analysis, the independent value of sNfL in the prediction of cognitive status was explored (Table 2). A correct classification of CI PwMS was found in 84.1% PwMS (specificity = 48.5%).
In a second model, imaging markers (i.e., NGMV, NLV and thalami), cNfL and cGFAP were included as predictors of cognitive status (N = 54), with only NGMV resulting as significant predictor of cognitive status (Table 2). The final model resulted in a sensitivity of 74.2%, a specificity of 65.2%, a PPV of 74.2%, a NPV of 65.2% and an accuracy of 70.4%. Both models yielded similar explained variances of ~ 25% (Table 2).
Multimodal marker for cognitive functioning
In a post-hoc analysis, the two significant predictors of cognitive status (NGMV and sNfL) were combined into a composite score as a multimodal marker for cognitive functioning in PwMS. Using the standardized betas as weights, the following formula was applied:
The AUC was larger for the composite score (AUC = 0.751, classification = fair), compared to NGMV (AUC = 0.696, classification = poor-sufficient) and sNfL (AUC = 0.680, classification = poor-sufficient). Classification of cognitive status using the composite score resulted in a sensitivity of 85.0%, a specificity of 57.6%, a PPV of 70.8%, a NPV of 76.0% and an accuracy of 72.6%. A higher composite score was associated with increased performance on multiple cognitive domains: processing speed (r = − 0.528, p < 0.001), verbal memory (r = − 0.436, p < 0.001), visuospatial memory (r = − 0.411, p < 0.001) and EF-verbal fluency (r = − 0.314, p = 0.008; Fig. 3).
Discussion
This study investigated the relation of NfL and GFAP measured in serum and CSF and cognitive performance in PwMS presenting with cognitive complaints, and their added predictive value compared to conventional imaging markers. Based on the levels of sNfL and sGFAP we were able to distinguish cognitively preserved from cognitively impaired PwMS, albeit with limited diagnostic accuracy. Increased levels of both serum NfL and GFAP were observed in cognitively impaired PwMS compared to cognitively preserved PwMS. NfL levels (in serum and CSF) were inversely associated with processing speed, indicating that decreased processing speed was associated with increased levels of sNfL and cNfL. No correlations could be detected between GFAP (measured in either serum or CSF) and cognitive functioning in PwMS. Finally, sNfL added unique variance in the prediction of cognitive status on top of NGMV. A composite score of both measures (a multimodal marker) resulted in a fair classification of cognitive status, stressing the need for a multimodal approach when predicting cognitive functioning.
Consistent with previous literature, increased levels of sNfL were found for cognitively impaired PwMS [7, 10, 11]. Furthermore, increased levels of sNfL and cNfL were associated with reduced processing speed. Slowed processing speed, has been hypothesized to be the major driver of cognitive impairment in MS [1], thereby possibly explaining why correlations with this specific domain are more prevalent in studies investigating NfL and cognitive functioning in MS [10, 12]. Yet, mixed results have been reported for increased levels of NfL and the performance in other cognitive domains [11]. Differences in sample size, administered neuropsychological tests, study population (i.e., a focus on newly diagnosed PwMS [12] or SPMS [8], a combination of MS types, or PwMS with mild cognitive impairment [39]) and specific focus on treatment are most likely explaining these differences [40]. In our sample, the distribution of PwMS on DMT at the time of the visit (but also the distribution of PwMS on first-line DMT vs. second-line DMT) was similar between cognitive groups, thereby reducing the likelihood of impacting our findings. Nonetheless, it could have played a role on an individual level as has been shown before [41]. Although it was beyond the scope of current research, the impact of DMTs on cognitive functioning in MS warrants further investigation [1]. Finally, although levels of sGFAP were increased in cognitively impaired PwMS, no correlations between GFAP and the cognitive domains survived correction for multiple comparisons, hereby limiting its potential as a clinical biomarker for cognitive functioning in MS.
Jakimovski et al. demonstrated in two previous studies a relatively weaker correlation between sNfL and cognition [10], compared to correlations between sNfL and MRI outcomes [42]. As potential explanation they put forward the role of adaptive processes to significantly influence the relationships between the released NfL and cognitive test results. Subsequently, PwMS who demonstrate preserved functional connectivity, despite ongoing structural pathology, can maintain high levels of cognitive performance [43]. In the current study, associations between sNfL and imaging markers were absent. However, associations of cNfL and sNfL between both processing speed and between cNfL and imaging markers were comparable in effect size with previous studies, thereby confirming aforementioned difference [10, 42]. Interestingly, in our study, imaging markers displayed a higher number of associations with multiple cognitive domains (not only processing speed, but also verbal and visuospatial memory) compared to fluid NfL and GFAP levels, highlighting that structural pathology was present and related to several cognitive test scores. As fluid biomarkers provide a real-time evaluation of the amount of pathology compared to the less dynamic imaging markers [9], it can be hypothesized that cognitive changes are not resulting from acute disturbances but rather from a more global effect over time on the brain in certain areas.
When added to the model, sNfL improved the prediction of cognitive status compared to NGMV alone. Especially when combining biomarkers, in our case NGMV and sNfL (the “multimodal marker”) a large effect was found for processing speed, whereas medium effects were reported for verbal and visuospatial memory. Even a medium sized effect for EF-verbal fluency was found when using the multimodal marker, which was absent when investigating individual markers. The current study is one of the first studies to combine both neuroimaging and fluid biomarkers of interest to detect cognitive impairment in MS. Investigating the role of a multimodal marker for cognitive functioning in PwMS is of high importance since these different modalities might reflect different aspects of neurodegeneration, which also has been reported in Alzheimer’s disease [44] and recently in MS as well [7, 45]. More specifically, previous studies investigating cross-modal fluid and imaging (bio)markers indeed show an “additive” effect of sNfL compared to cortical thickness [45] or lesion load and grey matter volume [7] in recently diagnosed PwMS. Together with our results, the added effect of sNfL highlights the necessity of using multiple sources of information to create a diagnostic marker for something as highly complex as cognitive performance, but also how these markers of neurodegeneration cannot be used interchangeably.
Nonetheless, clinical interpretation may be optimized when the full prognostic potential of sNfL for cognitive functioning will be evaluated over time, which is an important limitation of the current cross-sectional study design. The inclusion of a control group would have further aided the disentanglement between normal and abnormal levels of fluid and imaging (bio)markers. Also, contrary to measurements in serum, both NfL and GFAP measured in CSF were unable to discriminate between cognitive status. The most plausible explanation for this lack of detecting a difference is the limited power (N CSF = 54 versus N = 78 for serum). Performing a lumbar puncture is rather invasive and not all PwMS wanted to partake in this procedure. Importantly, without a post-contrast sequence being available in current study protocol, it was not possible to determine whether PwMS had active lesions at the time of evaluation. As a consequence, the investigation of the effect of recent disease activity on serum and CSF levels was limited and could be considered an important avenue for future research. Finally, the inclusion of a clinical, real-life sample is one of the biggest strengths, as the PwMS are reflective of our population at the outpatient clinic with perceived cognitive complaints. At the same time, being a real-life sample is also one of the main limitations. A homogenous sample is often desirable when investigating differences between groups, although data on other types of MS than RRMS is often lacking. Furthermore, given the fact that PwMS visited the outpatient clinic because of cognitive complaints, a slight bias towards cognitive impairment may have been present. The main clinical aim of the outpatient clinic is to investigate whether these complaints (or impairments) are due to MS pathology or, for instance, psychological or social factors (known to influence cognitive performance [46]). Results on PROMS measuring mood, anxiety, fatigue and sleep were, therefore, reported in this manuscript showing similar scores between cognitively preserved and impaired PwMS. Consequently, the impact of these factors on cognition was also considered similar.
In conclusion, we provided novel insights into the relationship between fluid biomarkers of neurodegeneration and their relation to cognitive functioning and conventional imaging measures in PwMS. The main finding of this study is the result that sNfL explains additional variance in cognitive performance on top of NGMV. A novel insight that was further explored in our study was the potential for combining two (bio)markers from a different origin when predicting cognitive status, instead of focusing on single measures of NfL or imaging outcomes. Combining multimodal biomarkers may be the way forward to enable timely identification of cognitive decline in MS.
Data availability
Anonymized data, not published in the article, can be shared upon reasonable request from a qualified investigator.
References
Benedict RHB, Amato MP, DeLuca J, Geurts JJG (2020) Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 19(10):860–871. https://doi.org/10.1016/S1474-4422(20)30277-5
Eijlers AJ et al (2018) Predicting cognitive decline in multiple sclerosis: a 5-year follow-up study. Brain 141(9):2605–2618
Schoonheim MM et al (2021) Disability in multiple sclerosis is related to thalamic connectivity and cortical network atrophy. Mult Scler J 28(1):61–70
Di Filippo M, Portaccio E, Mancini A, Calabresi P (2018) Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci 19(10):599–609
Zivadinov R et al (2016) Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 16(7):777–793
Stefano ND, Sormani MP (2020) Combining biomarkers to profile multiple sclerosis patients. Nat Rev Neurol 16(9):463–464
Brummer T et al (2022) Improved prediction of early cognitive impairment in multiple sclerosis combining blood and imaging biomarkers. Brain Commun 4(4):fcac153
Williams T et al (2022) Serum neurofilament light and MRI predictors of cognitive decline in patients with secondary progressive multiple sclerosis: analysis from the MS-STAT randomised controlled trial. Mult Scler J 28(12):1913–1926
Teunissen CE, Khalil M (2012) Neurofilaments as biomarkers in multiple sclerosis. Mult Scler J 18(5):552–556
Jakimovski D et al (2020) Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: a longitudinal retrospective 5-year study. Mult Scler J 26(13):1670–1681. https://doi.org/10.1177/1352458519881428
Ramani S, Berard JA, Walker LA (2021) The relationship between neurofilament light chain and cognition in neurological disorders: a scoping review. J Neurol Sci 420:117229
Gaetani L et al (2019) Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J Neurol 266(9):2157–2163
Högel H et al (2020) Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler J 26(2):210–219
Escartin C et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325. https://doi.org/10.1038/s41593-020-00783-4
Ayrignac X et al (2020) Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep 10(1):10923. https://doi.org/10.1038/s41598-020-67934-2
Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M (2018) Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep 8(1):14798. https://doi.org/10.1038/s41598-018-33158-8
Thompson AJ et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173
Schagen S, Schmand B, de Sterke S, Lindeboom J (1997) Amsterdam short-term memory test: a new procedure for the detection of feigned memory deficits. J Clin Exp Neuropsychol 19(1):43–51
Nauta I et al (2022) Performance validity in outpatients with multiple sclerosis and cognitive complaints. Mult Scler J 28(4):642–653. https://doi.org/10.1177/13524585211025780
Verhage F (1964) Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar. Van Gorcum, Assen
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1444
Benedict RH et al (2006) Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc JINS 12(4):549
Smith A (1982) Symbol digit modalities test (SDMT) manual (revised) Western Psychological Services, Los Angeles
Hammes J (1973) The STROOP color-word test: manual. Swets and Zeitlinger, Amsterdam
Mulder J, Dekker R, Dekker DH (1996) Verbale Leer- & Geheugen test: Handleiding [Verbal Learning & Memory Test: Manual]. Swets & Zeitlinger, Lisse
Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B (1996) Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychol Assess 8(2):145
Benton L, Hamsher K, Sivan A (1994) Controlled oral word association test, multilingual aphasia examination. AJA Associates, Iowa City
Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RH (2010) The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 16(1):6–16
Fischer M et al (2014) How reliable is the classification of cognitive impairment across different criteria in early and late stages of multiple sclerosis? J Neurol Sci 343(1–2):91–99
Benedict RH et al (2003) Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler J 9(1):95–101
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G (1994) Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 38(5):383–392
Soldatos CR, Dikeos DG, Paparrigopoulos TJ (2000) Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 48(6):555–560
Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CA (2010) Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reason Imaging 32(1):223–228. https://doi.org/10.1002/jmri.22214
Teunissen C et al (2009) A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 73(22):1914–1922
Kuhle J et al (2016) Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med (CCLM) 54(10):1655–1661
Hosmer DW Jr, Lemeshow S, Sturdivant RX (2013) Applied logistic regression. Wiley, New York
Nauta IM et al (2019) The clinical value of the patient-reported multiple sclerosis neuropsychological screening questionnaire. Mult Scler J 25(11):1543–1546. https://doi.org/10.1177/1352458518777295
Aktas O et al (2020) Serum neurofilament light chain: no clear relation to cognition and neuropsychiatric symptoms in stable MS. Neurol Neuroimmunol Neuroinflamm 7(6):e885. https://doi.org/10.1212/nxi.0000000000000885
Mattioli F et al (2020) Longitudinal serum neurofilament light chain (sNfL) concentration relates to cognitive function in multiple sclerosis patients. J Neurol 267(8):2245–2251. https://doi.org/10.1007/s00415-020-09832-1
Disanto G et al (2017) Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81(6):857–870
Jakimovski D et al (2019) Serum neurofilament light chain levels associations with gray matter pathology: a 5-year longitudinal study. Ann Clin Transl Neurol 6(9):1757–1770
Fuchs TA et al (2019) Preserved network functional connectivity underlies cognitive reserve in multiple sclerosis. Hum Brain Mapp 40(18):5231–5241
Ebenau JL et al (2022) Association of CSF, plasma, and imaging markers of neurodegeneration with clinical progression in people with subjective cognitive decline. Neurology 98(13):e1315–e1326
Cruz-Gomez ÁJ et al (2021) Cortical thickness and serum NfL explain cognitive dysfunction in newly diagnosed patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 8(6):e1074
Kalb R et al (2018) Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler J 24(13):1665–1680
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.v.D. is supported by a research grant from BMS. I.M.N. is supported by the Dutch MS Research Foundation, grant nr. 15-911. M.H. is supported by the Dutch MS Research Foundation, grant nr. 16-954b. J.J.G.G. has served as a consultant for or received research support from Biogen, Celgene, Genzyme, MedDay, Merck, Novartis and Teva. B.M.J.U. reports personal fees for consultancies from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva, outside the submitted work. C.E.T. has a collaboration contract with ADx Neurosciences and Quanterix, performed contract research or received grants from AC-Immune, Axon Neurosciences, Biogen, BioOrchestra, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, Grifols, Novo Nordisk, PeopleBio, Quanterix, Roche, Toyama, Vivoryon. She serves on editorial boards of Alzheimer Research and Therapy, and Neurology. H.E.H. serves on the editorial board of Multiple Sclerosis Journal, receives research support from the Dutch MS Research Foundation and the Dutch Research Council. She has served as a consultant for or received research support from Atara Biotherapeutics, Biogen, Novartis, Celgene/Bristol Meyers Squibb, Sanofi Genzyme, MedDay and Merck BV. B.A.d.J., E.A.W., M.K., B.M., and S.d.G.D. report no disclosures relevant to the manuscript.
Ethical standards statement
The Medical Ethics Research Committee of Amsterdam UMC concluded that the Medical Research Involving Human Subjects Act (WMO) did not apply to this study, as the data collection was part of clinical care (number METC-2016.395).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Dam, M., de Jong, B.A., Willemse, E.A.J. et al. A multimodal marker for cognitive functioning in multiple sclerosis: the role of NfL, GFAP and conventional MRI in predicting cognitive functioning in a prospective clinical cohort. J Neurol 270, 3851–3861 (2023). https://doi.org/10.1007/s00415-023-11676-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11676-4