Abstract
Introduction
Small vessel disease (SVD) causes most spontaneous intracerebral haemorrhage (ICH) and is associated with widespread microstructural brain tissue disruption, which can be quantified via diffusion tensor imaging (DTI) metrics: mean diffusivity (MD) and fractional anisotropy (FA). Little is known about the impact of whole-brain microstructural alterations after SVD-related ICH. We aimed to investigate: (1) association between whole-brain DTI metrics and functional outcome after ICH; and (2) predictive ability of these metrics compared to the pre-existing ICH score.
Methods
Sixty-eight patients (38.2% lobar) were retrospectively included. We assessed whole-brain DTI metrics (obtained within 5 days after ICH) in cortical and deep grey matter and white matter. We used univariable logistic regression to assess the associations between DTI and clinical-radiological variables and poor outcome (modified Rankin Scale > 2). We determined the optimal predictive variables (via LASSO estimation) in: model 1 (DTI variables only), model 2 (DTI plus non-DTI variables), model 3 (DTI plus ICH score). Optimism-adjusted C-statistics were calculated for each model and compared (likelihood ratio test) against the ICH score.
Results
Deep grey matter MD (OR 1.04 [95% CI 1.01–1.07], p = 0.010) and white matter MD (OR 1.11 [95% CI 1.01–1.23], p = 0.044) were associated (univariate analysis) with poor outcome. Discrimination values for model 1 (0.67 [95% CI 0.52–0.83]), model 2 (0.71 [95% CI 0.57–0.85) and model 3 (0.66 [95% CI 0.52–0.82]) were all significantly higher than the ICH score (0.62 [95% CI 0.49–0.75]).
Conclusion
Our exploratory study suggests that whole-brain microstructural disruption measured by DTI is associated with poor 6-month functional outcome after SVD-related ICH. Whole-brain DTI metrics performed better at predicting recovery than the existing ICH score.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous (non-traumatic) intracerebral haemorrhage (ICH) is a severe and frequently lethal or disabling form of stroke. ICH accounts for a minority (10–30%) of all strokes, but has substantial functional impact due to the high rate of residual disability in survivors [1]. Predicting functional outcome following ICH is challenging but essential to understand prognosis and optimize clinical care; this has led to great interest in predictive factors, models and composite scores to stratify the risk of death and functional recovery following ICH [2]. The most widely used and accepted prognostic score is the ICH score [3], which ranges from 0 to 6, including simple clinical and radiological variables (GCS score [2 point if GCS 3–4 and 1 point if GCS score 5–12], age [1 point if ≥ 80 years], ICH site [1 point for infratentorial origin], ICH volume [1 point if ≥ 30 cm3] and the presence of intraventricular haemorrhage [1 point if present]). The ICH score was designed to predict short-term (30-day) mortality and has limited predictive performance for longer-term functional outcome, with C-statistics ranging from 0.74 to 0.82 for 6-month functional outcome [4]. There is therefore still a need to find better prognostic instruments to determine the prognosis for recovery for each individual ICH patient.
Diffusion tensor imaging (DTI) can quantify and map microarchitectural integrity and structural connectivity in white and grey matter in vivo, based upon water molecular motion directional preference (captured by fractional anisotropy, FA) and magnitude (captured by mean diffusivity, MD). Little is known about the impact of whole-brain microstructural alterations after SVD-related ICH; most previous studies have used an operator-dependent ROI-based approach to predict functional outcome based on FA and MD variations in the corticospinal tract (CST) after non-lobar (deep) ICH [5]. However, whole-brain DTI analysis is emerging as a new potential marker of overall cerebrovascular burden, being associated with structural SVD MRI biomarkers, cognitive decline and dementia [6, 7]. Small vessel disease (SVD), responsible for nearly 85% of spontaneous ICH [8], could plausibly lead to disruption of key brain networks involved in rehabilitation, learning, motor recovery and cognitive reserve [9, 10] with potential relevance for functional recovery. Other factors that could influence tissue microstructure after ICH include vasogenic oedema, early effects of Wallerian degeneration (which appear within days of the lesion in animal models), inflammation and acute disruption of fibres from ICH-related pressure. Whole-brain DTI has not yet been applied to predict 6-month outcome after acute ICH. In this exploratory study of a cohort of mixed (lobar and non-lobar) ICH patients, we aimed to investigate: (1) the associations between whole-brain DTI metrics—as biomarkers of SVD-related widespread injury—and 6-month functional outcome after acute ICH; and (2) the predictive ability of these metrics compared to the pre-existing clinical-radiological ICH score.
Methods
Study population
We retrospectively included consecutively recruited patients with first-ever spontaneous (non-traumatic) ICH attributed to cerebral SVD (after exclusion of macrovascular and structural causes) included in the prospective SIGNAL (Stroke InvestiGation in North And Central London) registry from January 2017 to March 2019. Inclusion criteria were the availability of adequate quality DTI-MRI within 5 days of ICH and follow-up for the mRS at 6 months. We included patients with early (< 5 days) DTI availability for two reasons: first, to include a homogeneous population in terms of pathophysiological changes occurring after ICH; and second, to explore DTI metrics’ predictive ability in the early acute phase of care, where outcome prediction is likely to be most valuable to guide clinical management. From the original cohort of patients with diagnosis of any SVD-related ICH who had an MRI as part of standard care (N = 200), we included 68 patients (34%); we excluded 132 patients (66%): 20 (10%) patients were lost to follow-up, 26 (13%) underwent MRI after 120 h (5 days) from the acute ICH index event symptom onset, and 86 (43%) had no DTI-MRI available (Fig. 1).
Clinical evaluation
We retrieved baseline detailed demographic, clinical and radiological information from the SIGNAL database and electronic medical records. Six-month functional outcome was assessed via modified Rankin Scale (mRS) [11] at follow-up visits or by phone call. Data were collected as part of routine clinical care, and data analysis was approved as a service evaluation by the University College London Hospital Trust Data Governance Review Board (service evaluation 5-201920-SE).
MRI acquisition
All MRI scans were performed on a Philips Achieva 3 Tesla scanner (Philips, Best, Netherlands). The following acquisitions were included: diffusion-weighted imaging (DWI) (voxel resolution 0.9 × 0.9 × 5 mm3, echo time 76 ms, repetition time 3.5 s, flip angle 90°) comprising one b = 0, and 6 b = 1000 s/mm2 volumes; T1-weighted imaging (voxel resolution 0.94 × 0.94 × 1.1 mm3, echo time 3.3 ms, repetition time 7.1 ms, flip angle 9°); fluid-attenuated inversion recovery (FLAIR) imaging (voxel resolution 0.45 × 0.45 × 4 mm3, echo time 110 ms, repetition time 10.8 s, inversion time 2.8 s, flip angle 90°); and susceptibility-weighted imaging (SWI) (voxel resolution 0.24 × 0.24 × 1 mm3, repetition time 31 ms, flip angle 17°).
MRI analysis and lesion segmentation
ICH location was assessed using the Cerebral Haemorrhage Anatomical Rating Instrument (CHARTS) [12]. The ICH score was calculated for every patient according to the original publication [3]. Every MRI was evaluated by a single rater (GS) blinded to other clinical variables. ICH and peri-haematomal oedema (PHE) regions were manually segmented on SWI and FLAIR sequences, respectively. The regions of interest (ROI) obtained from ICH and PHE segmentations were used to obtain the ICH and PHE volumes.
MRI image processing and DTI metrics
DWI data were corrected for eddy currents and patient motion and DTI-fitted using FSL [13]. To obtain the ICH and PHE ROIs in each subject’s DWI space, non-rigid transformations were computed between SWI and DWI, and between FLAIR and DWI image spaces. All image transformations were done using the NiftyReg software package [14]. Mean FA and MD were computed in three brain compartments: cortical grey matter, deep grey matter and white matter (obtained using the Geodesic Information Flows brain segmentation algorithm, available at http://niftyweb.cs.ucl.ac.uk/) [15]. The anatomical regions included in each brain compartment are listed in Supplementary material. All DTI metrics were obtained in five different settings: in unaffected hemisphere (1), in affected hemisphere with (2) and without (3) ICH lesion masked out and in the entire brain with (4) and without (5) lesion masked out. Haemorrhagic lesion probability map was computed using a non-rigid transformation of each brain to the MNI space using NiftyReg software packages. Then, all the lesion masks were summed and divided by the number of subjects to obtain the final lesion probability map.
Statistical analysis
Poor 6-month functional outcome was defined as mRS greater than 2 for patients with pre-ICH mRS 0–2; for patients with pre-ICH mRS > 2, poor 6-month outcome was considered any worsening in the mRS score between the pre-morbid mRS and the 6-month mRS (i.e. a return to baseline pre-ICH mRS was considered good functional outcome). We described our cohort with frequencies and percentages for categorical variables and using mean and standard deviation (SD) or median with interquartile range (IQR) for continuous variables.
We assessed the univariable association between 6-month functional outcome with DTI and non-DTI variables (clinical and standard radiological variables) using logistic regression analysis. To avoid unnecessary predictors, we assessed DTI variables’ correlation (Pearson correlation) with the intent to exclude DTI variables found to have a very high correlation with each other (r > 0.9). To select variables for a multivariable model, we selected DTI and non-DTI predictors with p < 0.10 in univariable logistic regression analyses and fitted logistic regression models using least absolute shrinkage and selection operator (LASSO) estimation to avoid overfitting [16]. The best predictors were identified in three different models: model 1 included only DTI variables, model 2 combined DTI plus non-DTI variables and model 3 with DTI variables plus ICH score. We validated the models using bootstrapping, and calculated optimism-adjusted estimates of the AUC. To compare the predictive abilities of model 1, model 2 and model 3 against the ICH score, we re-fitted the selected LASSO models (using standard logistic regression—including the ICH score, to obtain nested models) and compared them using likelihood ratio (LR) tests.
In our primary analysis, we evaluated DTI metrics in the whole brain, including the ICH lesion (setting 5) therefore not requiring operator intervention. In sensitivity analyses, we explored the predictive ability of DTI metrics in the other settings: (1) whole brain excluding haemorrhagic lesion (ICH masked out), (2) DTI metrics in the unaffected hemisphere only, (3) DTI metrics in the affected hemisphere only, with (3a) and without (3b) haemorrhagic lesion masked out. Moreover, we performed another sensitivity analysis to assess association and outcome predictive ability of DTI metrics (in the whole brain including ICH lesion), for 6-month excellent outcome (mRS 0–1).
We also performed mass-univariate, voxel-wise analyses using FSL [13], and assessed whether any brain areas had significantly different DTI metrics in patients with poor versus good 6-month functional outcome.
Statistical analyses were performed using STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LP) for numerical data and FSL for voxel-wise analysis [13]. The significance level was set at p = 0.05.
This study is conducted following the TRIPOD guidelines [17].
Results
We included 68 consecutive eligible patients: Table 1 summarizes their clinical and radiological characteristics including DTI metrics. Twenty-one patients (30.9%) had poor 6-month mRS.
In univariable logistic regression analysis (Table 2) ICH volume (OR 1.66 [95% CI 1.00–2.74]; p = 0.048) and intra-ventricular extension (IVH) (OR 9.03 [95% CI 2.09– 39.02], p = 0.003) were associated with poor mRS. We found no DTI variables to have correlation r > 0.9 with each other (Table E1, Supplementary material). Among DTI metrics (Table 2), deep grey matter MD (OR 1.04 [95% CI 1.01–1.07], p = 0.010) and white matter MD (OR 1.11 [95% CI 1.01–1.23], p = 0.044) were significantly associated with 6-month mRS.
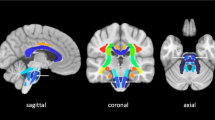
Figure 2 shows a threshold-free, cluster-enhanced, voxel-wise correlation map highlighting brain areas where MD was significantly greater (in red-yellow) or FA significantly lower (in green) (familywise error corrected p < 0.05 for both) in patients with poor versus good 6-month outcome, overlaid on the MNI152 1 mm brain atlas alongside a haemorrhagic lesion probability map (blue-white scale); an additional voxel-based analysis performed by swapping all affected hemispheres to the right side of the MNI152 brain is reported as supplementary material (Figure S1).
Threshold-free, cluster-enhanced, voxel-wise correlation map showing brain areas where MD was significantly greater (in red-yellow) or FA significantly lower (in green) (familywise error corrected p < 0.05 for both) in patients with poor versus good functional outcome, overlaid on the MNI152 1 mm brain atlas alongside a haemorrhagic lesion probability map (blue-white scale, range 5–20%)
The predictive ability for the ICH score alone and all models including DTI data is shown in Table 3. The discrimination values were as follows: ICH score alone 0.62 (95% CI 0.49–0.75); model 1 (including deep grey matter MD) 0.67 (95% 0.52–0.83); model 2 (including deep grey matter MD, ICH volume and IVH) 0.71 (95% CI 0.57–0.85); and model 3 (including deep grey matter MD and ICH score) 0.66 (95% CI 0.52–0.82). All the models including DTI parameters had statistically significant superior performance compared to the ICH score alone (LR tests for models 1, 2 and 3 compared to the ICH score alone gave p-values of 0.031, 0.002 and 0.034, respectively).
As reported in Supplementary Tables E3, sensitivity analyses (including DTI metrics in different settings [whole brain with the ICH masked out, affected and unaffected hemispheres] led to consistent results: deep grey matter MD was consistently associated with poor outcome and selected as best predictive variable via LASSO regression analyses; all models including DTI metrics had statistically significant superior performances compared to ICH score alone. DTI metrics were also associated with a poor outcome defined as mRS > 1 (rather than mRS > 2) with AUCs ranging from 0.62 to 0.68, although these models did not significantly improved outcome prediction in comparison with the ICH score alone (AUC 0.61[95% CI 0.50–0.71]; Supplementary Table E4).
Discussion
Our exploratory study in ICH survivors showed that acute whole-brain DTI metrics (deep grey matter MD) are associated with 6-month functional outcome, providing new insight into the relevance of SVD-related microstructural changes after ICH. The predictive performance of whole-brain DTI metrics, alone or in combination with other clinical-radiological variables, offers improved functional outcome prediction compared to the existing ICH score, regardless of the use of ICH lesion segmentation. Since whole-brain DTI metrics can be quantified without any operator intervention, they have potential to be applied in everyday clinical practice, being also a potential target for future therapeutic interventions.
Most of the studies on the application of DTI in ICH mainly focus on microstructural damage quantification within a specific fibre tract (mainly the corticospinal tract, CST), directly related to the index haemorrhagic event. The limited available data are heterogeneous, leading to conflicting results [5]. Nevertheless, DTI seems feasible and of potential clinical predictive value in subacute ICH [18, 19]. Besides the application on specific fibre tract (directly injured from ICH), whole-brain objective DTI measures might be a valuable marker of diffuse microstructural brain injury due to pre-existing small vessel disease (SVD) [6]. Indeed, fully automated whole-brain DTI approaches showed excellent correlation with cognition, outperforming conventional MRI biomarkers of SVD (white matter hyperintensities, lacunes, brain volume, cerebral microbleeds and perivascular spaces) in predicting cognition in hereditary and sporadic variants of SVD [6, 7, 20, 21]. Therefore, whole-brain DTI parameters have been proposed as a convenient marker of overall cerebrovascular burden in patients with SVD, being able to monitor disease progression, assess therapeutic intervention and predict cognitive decline and dementia [22]. Most spontaneous (non-traumatic) ICH are caused by SVD[23, 24], mainly arteriolosclerosis (deep perforator arteriopathy) and cerebral amyloid angiopathy [25]. To the best of our knowledge, this is the first attempt to quantifying widespread SVD-related microstructural damage via a whole-brain DTI approach after ICH due to SVD. Our results indicate that DTI can quantify widespread microstructural tissue alterations relevant for functional outcome prediction after deep or lobar ICH. The changes in MD we found in patients with poor outcome might in part be related to whole-brain SVD burden; increasing evidence suggests that inflammation, endothelial dysfunction, blood–brain barrier injury and microglial activation [26,27,28] are involved in the pathogenesis of SVD. Other potential contributors to MD in acute ICH include disruption of the blood–brain barrier, leading to water extravasation and vasogenic oedema [28], the early effects of Wallerian degeneration, inflammation, and acute disruption of fibres from ICH-related pressure. All of these acute-phase changes may lead to an increase in the total water content of the tissue with consequent elevation in MD.
Regardless of the site of measurement, we found MD to be more strongly associated with functional outcome than FA. We acknowledge that to perform MRI early after ICH may have had an impact on our finding, with recent data [29] suggesting that FA may not be such a sensitive marker of ICH-induced microstructural changes when acquired in the acute phase after ICH. In general, FA measures the degree to which water diffuses in one main direction, with increased anisotropy in the presence of highly oriented fibres. Although when assessing the integrity of a specific fibre tract, FA is an appropriate metric [8], FA might be less meaningful with a whole-brain approach which simultaneously includes white matter fibres with different orientation as well as grey matter structures. Furthermore, fibre tract anisotropy loss (relevant for functional recovery after stroke) might be mainly due to Wallerian degeneration [27], which may take more than 5 days to develop. Conversely, MD measures the degree of restriction of diffusion of water molecules, independent of direction, so in a whole-brain approach might be the best metric to widespread microstructural disruption due to both neuronal or white matter tissue injury. Deep grey matter mean diffusivity had the optimal predictive value of the whole-brain DTI metrics investigated in our study. Given the absence of similar studies, this finding requires confirmation in other cohorts and cannot be critically compared with other available data. This result should be interpreted considering the high prevalence of deep supratentorial ICH in our cohort (60.3% vs. 38.2% lobar ICH). Deep ICH can be considered primarily due to arteriolosclerosis (deep perforator arteriopathy (DPA)) which preferentially affects deep grey matter structures [25].
Our study has several strengths. To the best of our knowledge, ours is the first and largest study to quantify widespread whole-brain microstructural injury in a mixed population of spontaneous acute lobar and deep ICH due to SVD. We used LASSO estimation—a technique that produces better models for prediction in small datasets. We also applied a standardized automated DTI post-processing protocol. We must also acknowledge several limitations. Our study has a retrospective design with a small sample size, so our findings are exploratory and should be confirmed in larger independent cohorts. We used 6-direction and 3 Tesla DTI-MRI: this influenced the quality of the DTI data in comparison with acquisitions with more diffusion gradient directions; however, to increase the number of directions would increase the scanning time, consequently increasing motion artefacts and ultimately affecting the chances to apply DTI sequences in real-world acute strokes’ environment. Indeed, the requirement for MRI could have created a selection bias to less severe ICH survivors (with only one patients having infratentorial ICH, included as a poor prognostic factor in the ICH score), which could limit the generalizability of our results: the patients in our cohort are relatively young (median age 66.5 years [IQR 55.5–75.5]), have no prior history of stroke and had small ICH volumes (median volume 7.1 ml [IQR 3.2–13.7]). We found that although DTI metrics were associated with excellent (6-month mRS 0–1) outcome prediction, they did not statistically improve prediction over the ICH score alone. Despite no major differences in terms of pre-ICH mRS between included and excluded patients (median 0 [IQR 0–1] versus median 0 [IQR 0–1]; p = 0.091), included patients had lower 6-month mRS (median 2 [IQR 1–3] versus median 3 [IQR 1–4]; p = 0.010). Nevertheless, prognostic information is valuable in ICH survivors able to undergo MRI as part of routine care for planning of ongoing stroke care and rehabilitation pathways. Further studies should ideally include more severely affected patients, but participation may remain limited due to the feasibility of acquiring MRI in the acute phase of ICH.
Conclusion
Our exploratory study suggests that whole-brain DTI diffusivity metrics assessing microstructural alterations are associated with 6-month functional outcome after ICH due to SVD and might perform better at predicting recovery than the existing clinical-radiological ICH score alone.
Data sharing and availability statement
All de-identified participant data requests should be submitted to the corresponding author for consideration by the SIGNAL Steering Committees.
References
Moulin S, Cordonnier C (2015) Prognosis and outcome of intracerebral haemorrhage. Front Neurol Neurosci 37:182–192
Hammerbeck U, Abdulle A, Heal C, Parry-Jones AR (2022) Hyperacute prediction of functional outcome in spontaneous intracerebral haemorrhage: systematic review and meta-analysis. Eur Stroke J 7(1):6–14
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32(4):891–897
Gregorio T, Pipa S, Cavaleiro P, Atanasio G, Albuquerque I, Chaves PC et al (2019) Assessment and comparison of the four most extensively validated prognostic scales for intracerebral hemorrhage: systematic review with meta-analysis. Neurocrit Care 30(2):449–466
McGurgan IJ, Ziai WC, Werring DJ, Al-Shahi Salman R, Parry-Jones AR (2020) Acute intracerebral haemorrhage: diagnosis and management. Pract Neurol 21(2):128–136
Wardlaw JM, Smith C, Dichgans M (2019) Small vessel disease: mechanisms and clinical implications. Lancet Neurol 18(7):684–696
Valdes Hernandez Mdel C, Booth T, Murray C, Gow AJ, Penke L, Morris Z et al (2013) Brain white matter damage in aging and cognitive ability in youth and older age. Neurobiol Aging 34(12):2740–2747
Moura LM, Luccas R, de Paiva JPQ, Amaro E Jr, Leemans A, Leite CDC et al (2019) Diffusion tensor imaging biomarkers to predict motor outcomes in stroke: a narrative review. Front Neurol 10:445
Low A, Mak E, Stefaniak JD, Malpetti M, Nicastro N, Savulich G et al (2020) Peak width of skeletonized mean diffusivity as a marker of diffuse cerebrovascular damage. Front Neurosci 14:238
Baykara E, Gesierich B, Adam R, Tuladhar AM, Biesbroek JM, Koek HL et al (2016) A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol 80(4):581–592
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19(5):604–607
Charidimou A, Schmitt A, Wilson D, Yakushiji Y, Gregoire SM, Fox Z et al (2017) The Cerebral Haemorrhage Anatomical RaTing inStrument (CHARTS): development and assessment of reliability. J Neurol Sci 372:178–183
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62(2):782–790
Modat M (2012) Inverse-consistent symmetric free form deformation. In: Dawant BITM, Christensen GE, Fitzpatrick JM, Rueckert D (eds) Biomedical image registration. Springer, Berlin Heidelberg, pp 79–88
Cardoso MJ, Modat M, Wolz R, Melbourne A, Cash D, Rueckert D et al (2015) Geodesic information flows: spatially-variant graphs and their application to segmentation and fusion. IEEE Trans Med Imaging 34(9):1976–1988
Ambler G, Brady AR, Royston P (2002) Simplifying a prognostic model: a simulation study based on clinical data. Stat Med 21(24):3803–3822
Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 162(1):55–63
Schwarz G, Kanber B, Prados F, Browning S, Simister R, Jager R et al (2022) Acute corticospinal tract diffusion tensor imaging predicts 6-month functional outcome after intracerebral haemorrhage. J Neurol 269(11):6058–6066
Chang MC, Kwak SG, Park D (2021) Prediction of the motor prognosis with diffusion tensor imaging in hemorrhagic stroke: a meta-analysis. J Integr Neurosci 20(4):1011–1017
Raposo N, Zanon Zotin MC, Schoemaker D, Xiong L, Fotiadis P, Charidimou A et al (2021) Peak width of skeletonized mean diffusivity as neuroimaging biomarker in cerebral amyloid angiopathy. AJNR Am J Neuroradiol 42(5):875–881
Konieczny MJ, Dewenter A, Ter Telgte A, Gesierich B, Wiegertjes K, Finsterwalder S et al (2021) Multi-shell diffusion MRI models for white matter characterization in cerebral small vessel disease. Neurology 96(5):e698–e708
Zeestraten EA, Lawrence AJ, Lambert C, Benjamin P, Brookes RL, Mackinnon AD et al (2017) Change in multimodal MRI markers predicts dementia risk in cerebral small vessel disease. Neurology 89(18):1869–1876
Hilkens NA, van Asch CJJ, Werring DJ, Wilson D, Rinkel GJE, Algra A et al (2018) Predicting the presence of macrovascular causes in non-traumatic intracerebral haemorrhage: the DIAGRAM prediction score. J Neurol Neurosurg Psychiatry 89(7):674–679
Hostettler IC, Seiffge DJ, Werring DJ (2019) Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother 19(7):679–694
Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9(7):689–701
Puig J, Blasco G, Terceno M, Daunis IEP, Schlaug G, Hernandez-Perez M et al (2019) Predicting motor outcome in acute intracerebral hemorrhage. AJNR Am J Neuroradiol 40(5):769–775
Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH et al (2000) Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 69(2):269–272
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD (2019) Global brain inflammation in stroke. Lancet Neurol 18(11):1058–1066
Low A, Mak E, Rowe JB, Markus HS, O’Brien JT (2019) Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev 53:100916
Funding
This work was undertaken at UCLH/UCL which receives a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the manuscript. GS, BK, FP, GA, CAMWK and DJW contributed to the design of the study, analysis and interpretation of data and drafting the manuscript for intellectual content. SB, RS and RJ played a major role in acquisition of data and revising manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
DJW received funding from the Stroke Foundation/British Heart Foundation. RS, FP and BK received funding from UCLH/UCL BRC. The remaining authors declare no financial or other conflicts of interest.
Ethics approval
The SIGNaL registry (which contains routinely collected clinical data) is approved by the University College Hospitals NHS Foundation Trust Governance Review Board as a continuous service evaluation of a comprehensive clinical care program (service evaluation 5-201920-SE); for this reason, informed patient consent was not required.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwarz, G., Kanber, B., Prados, F. et al. Whole-brain diffusion tensor imaging predicts 6-month functional outcome in acute intracerebral haemorrhage. J Neurol 270, 2640–2648 (2023). https://doi.org/10.1007/s00415-023-11592-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11592-7