Abstract
Objective
To examine whether associations between individual neuropsychiatric symptoms (NPS) and incident Alzheimer’s dementia (AD) differ in men versus women.
Methods
Data were acquired from the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set. Two sets of older (≥ 60 years) participants were formed: one of cognitively unimpaired (CU) individuals, and one of participants with mild cognitive impairment (MCI). NPS were assessed using the Neuropsychiatric Inventory Questionnaire. Cox proportional hazards models examined associations between individual NPS and AD incidence separately for each participant set. These models featured individual NPS, sex, NPS by sex interactions as well as a number of covariates.
Results
The analysis involved 9,854 CU individuals followed for 5.5 ± 3.8 years and 6,369 participants with MCI followed for 3.8 ± 3.0 years. NPS were comparably associated with future AD in men and women with MCI. Regarding CU participants, the following significant sex by NPS interactions were noted: female sex moderated the risk conferred by moderate/severe apathy (HR = 7.36, 3.25–16.64) by 74%, mitigated the risk conferred by moderate/severe depression (HR = 3.61, 2.08–6.28) by 52%, and augmented the risks conferred by mild depression (HR = 1.00, 0.60–1.68) and agitation (HR = 0.81, 0.40–1.64) by 83% and 243%, respectively.
Conclusions
Apathy, depression and agitation were differentially associated with incident AD in CU men and women. No individual NPS was associated with different risks of future AD in men versus women with MCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropsychiatric symptoms (NPS) are almost universal among older adults with mild cognitive impairment (MCI) and Alzheimer’s dementia (AD) [1, 2]. In cognitively unimpaired (CU) individuals, NPS have been linked to more precipitous cognitive decline [3] and elevated risk of incident MCI or AD [4, 5]. Further, NPS have been associated with a greater risk of progression from MCI to AD [6] and steeper cognitive trajectories in patients with AD [7]. Therefore, regardless of the exact underlying pathophysiological connection, the presence of NPS in older adults is a predictor of worse cognitive impairment and AD.
Biological sex appears to modify the course of AD. Different sex-related associations have been reported for prognosis, risk factors and drug effects (to name a few aspects of sex interactions) [8]. However, sex effects have received limited attention in NPS research. Some have reported substantial differences in NPS between men and women: women present with a broader range of NPS and carry a greater neuropsychiatric burden compared to men [9]. Further, psychotic and affective symptoms afflict women more frequently, whereas apathy appears to be more prevalent among men with AD [10].
Despite the evidence of sex-specific NPS patterns in AD, the predictive value of NPS has not been explored separately in men and women. Although psychotic and affective symptoms, apathy, agitation, irritability, and aberrant motor behaviour have been related to faster progression from normal aging to MCI and ultimately AD, it is not clear whether these associations differ between men and women [5, 11,12,13,14]. The aim of the present study was to examine the predictive properties of NPS separately in male and female older adults. We capitalised on longitudinal data from the Uniform Data Set (UDS), a set of prospectively collected data on volunteers from multiple Alzheimer's disease research centres (ADRCs) across the United States.
Methods
UDS is a central repository of collaborative-multidisciplinary data collected in National Institute on Aging/NIH—funded ADRCs across the United States stewarded by the National Alzheimer's Coordinating Center (NACC). The key methodological features of the UDS have been detailed elsewhere [15,16,17]. In brief, it was initiated in 2005 and operates to date, as a resource for dementia research. Participants are recruited according to each ADRC’s distinct protocol and undergo standardized evaluations on an approximately annual basis. Participants or surrogates provide informed consent before participation. All procedures are overseen by Institutional Review Boards at each ADRC and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Eligibility criteria and participant selection
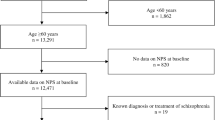
The current study was based on data from the subset of older (≥ 60 years) NACC participants enrolled between September 2005 (inception of the UDS) and December 2021 (data freeze) from a total of 43 ADRCs. Two distinct groups of individuals were assembled. The first involved every individual who was CU at entry to the NACC (CU set). The second included individuals diagnosed with MCI at entry to the NACC plus those who developed MCI at follow-up, without a prior dementia diagnosis (MCI set). For those who developed MCI at follow-up, the first visit at which they were diagnosed with MCI was the starting point of their monitoring. Participants with a follow-up diagnosis of dementia other than AD were excluded (to avoid competing risks of conversion to non-AD dementias).
Cognitive diagnoses were established by either an interdisciplinary consensus team (in the majority of cases) or a single clinician (who conducted the examination), depending on the specific requirements of each ADRC’s protocol. Diagnoses were based on medical history, neuropsychological performance, and psychosocial functioning. CU was defined by the absence of a diagnosis of dementia, MCI or cognitive impairment not MCI, according to the physician-based diagnosis. MCI and dementia were diagnosed using standard clinical criteria [18,19,20,21,22,23]. Participants with cognitive impairment who did not clearly fit into the categories of CU, MCI, or dementia were diagnosed as cognitively impaired, not MCI.
Individuals reporting treatment with an FDA-approved medication for AD (i.e. tacrine, donepezil, rivastigmine, galantamine and memantine) were excluded from both sets (receiving such medication raised doubts about the credibility of the clinician-based diagnoses). Participants with a physician’s diagnosis of a psychiatric disorder (schizophrenia, bipolar disorder, post-traumatic stress disorder, obsessive–compulsive disorder and developmental neuropsychiatric conditions) were also excluded from both groups to eliminate the confounding of long-standing neuropsychiatric manifestations that interfere significantly with cognition.
Measurement of NPS
The Neuropsychiatric Inventory Questionnaire (NPI-Q) is an informant administered, widely used tool for the evaluation of NPS in dementia research [24]. NPI-Q evaluates 12 domains: delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviour, night-time behaviours, and eating behaviours. Informants initially report the presence or absence of cardinal symptomatology for each domain in the month preceding the examination and subsequently rate the severity of any symptoms according to a 3-point severity scale: mild (noticeable, but not a significant change); moderate (significant, but not a dramatic change); or severe (very marked or prominent; a dramatic change). For most NPI-Q domains, participants were grouped according to NPS on a 3-point scale: 0: absent; 1: mild; 2: moderate and severe symptomatology (e.g., irritability: absent, mild, moderate/severe; anxiety: absent, mild, moderate/severe and so on). For the domains of delusions, hallucinations, elation/euphoria and aberrant motor behaviour, owing to the very small number of participants with moderate/severe symptomatology, participants were dichotomized for presence of these NPS (0: absent; 1: mild, moderate and severe symptomatology) [25].
Factors and covariates considered
Age at the time of the baseline evaluation and education in years of formal schooling were treated as scale variables. Sex, race (Caucasian, African American, American Indian or Alaska Native, Native Hawaiian or Pacific Islander, Asian and multiracial), number of apoE4 alleles (0 or 1 or 2), as well as a number of comorbidities, medications and habits that may confound the relationship between NPS and cognitive decline were treated as categorical variables: history of seizures, traumatic brain injury (TBI), Parkinson’s disease (PD), cerebrovascular disease (CEVD), cardiovascular disease (CAVD), diabetes mellitus (DM), hypertension, dyslipidaemia, smoking history, alcohol abuse or other substance abuse (with clinically significant impairment occurring over a 12-month period manifested in one of the following areas: work, driving, legal, or social), vitamin B12 deficiency, reported use of antidepressants, reported use of antipsychotics and reported use of anxiolytic/sedative/hypnotic agents. These parameters were positively assessed according to subject or co-participant reporting. To avoid over-adjustment, a statistical criterion was set for the inclusion of each factor in our analysis: only covariates that significantly differed between those without and those with at least one NPS were included (age, education, sex and race were accounted for regardless of the statistical prerequisite). Therefore, different sets of covariates were considered for the CU and MCI sets.
Statistical analysis
Baseline differences between those who did and those who did not progress to AD were analysed using independent sample t-tests (scale variables) and Pearson’s chi-squared tests (categorical variables). Results are provided separately for the CU and MCI sets.Please confirm the section headings are correctly identified.I confirm that the headings are correctly identified
Associations between NPS and incident AD were examined using multivariable adjusted Cox proportional hazards models. Twelve separate, independent analyses were performed for each participants set, one analysis per NPS. Participants were censored at their last visit. The proportionality of hazards for different strata over time was confirmed for each model using cox regression analyses with time dependent covariates. For example, to test the proportionality of hazards for anxiety, an extended Cox model including the term anxiety*time along with anxiety was analysed. To verify that the proportionality of hazards assumption was not violated, the coefficient of the time interaction product had to be statistically insignificant.
Regarding the CU set, all analyses featured the following covariates (based on the aforementioned statistical criterion): age, education, race, PD, CEVD, CAVD, DM, hypertension, dyslipidaemia, smoking history, alcohol or other substance abuse, B12 deficiency, reported use of antidepressants, antipsychotics, or anxiolytic/sedative/hypnotic agents and sex, along with one NPS at a time (e.g., anxiety) including sex by NPS interaction terms (e.g., sex*anxiety). In the case of MCI, all 12 survival analyses featured the following covariates (based on the statistical prerequisite): age, education, race, PD, TBI, number of apoE4 alleles, alcohol or other substance abuse, B12 deficiency, reported use of antidepressants, antipsychotics or anxiolytic/sedative/hypnotic agents and sex, along with one NPS at a time (e.g., anxiety) including sex by NPS interaction terms (e.g., sex*anxiety).
To ascertain the validity of our findings, confirmatory analyses were conducted accounting for the potential confounding of preclinical cognitive alterations [26]: analyses were repeated after adjusting for baseline mini-mental state examination (MMSE) scores, as a measure of global cognition [27, 28]. To limit the amount of missing data, results from the NACC neuropsychological battery crosswalk study were utilised [29]. In specific, Montreal Cognitive Assessment (MoCA) scores were converted to equivalent MMSE scores according to the detailed conversion tables provided by the crosswalk study investigators (although the first two versions of the UDS assessed global cognition using MMSE, the more recent, third version of the UDS replaced MMSE with MoCA) [30, 31].
Statistical analyses were performed using the IBM SPSS Statistics Software Version 27 (Chicago, IL, USA). Despite performing multiple comparisons, the conventional threshold of α = 0.05 was implemented for the revelation of statistical significance. This decision was made to retain a fair statistical power for our analyses, because the great number of factors and covariates featured in our models along with the low frequency of several investigated exposures-NPS strata (e.g., delusions, hallucinations, moderate-severe depression, disinhibition, and so on) considerably undermined precision estimates (there was a risk of failing to reveal valid associations).
Results
CU participant characteristics and missing data
Of the 44,713 participants in UDS, 11,018 CU at baseline were eligible for the analysis (Fig. 1). A total of 621 were not included due to missing data on covariates. An additional 543 were not included in any model because of missing data on all NPS. Of the remaining 9854, between 0 and 11 individuals were excluded from each survival analysis (due to missing data on the specifically analysed NPS), with the exception of night-time behaviours (68 cases with missing data were excluded).
CU individuals with missing data (n = 1,164) were older (74.5 ± 8.5 vs. 73.0 ± 7.6 years), less educated (15.4 ± 3.4 vs. 15.9 ± 2.9 years) and more often African American or Asian compared to those without missing data (who were more often white). CEVD, DM, hypertension, night-time behaviours, depression, anxiety and irritability were more prevalent among those with missing data, while the use of antidepressants was less common (missing data analysis not shown).
Throughout the average follow-up of 5.5 ± 3.8 years (range 0.4–15.9 years), 643 older CU adults progressed to AD while 9211 did not. Baseline differences between those who did and did not develop AD are in Table 1.
NPS and incident AD in CU individuals
While many NPS were associated with an increased hazard of AD, the main effect of sex was not significant in any of the 12 survival analyses (Table 2, main effects). In most NPI-Q domains, NPS were comparably associated with future AD in men and women (Table 2, main effects): e.g., mild irritability was linked a two-fold hazard (HR = 2.01) of incident AD, while moderate-severe irritability was related to almost four-fold hazard (HR = 3.89) in both sexes. However, in the case of apathy, depression and agitation there were significant sex by NPS interactions. Moderate/severe apathy was linked to 7.36 greater overall risk of AD, but female sex substantially moderated this effect: women with moderate/severe apathy had one quarter (effect size = 0.26) of the aforementioned hazard (Fig. 2). Moderate-severe depression was related to 3.61 greater risk of progression to AD. Women with moderate/severe depression had only half (effect size = 0.48) this hazard. On the other hand, mild depression conferred an increased risk of AD only in women (female sex augmented the hazard by 83%) (Fig. 3). As for moderate-severe agitation, it was linked to an elevated hazard of progression to AD in both sexes (HR = 3.36), whereas mild symptoms conferred a greater hazard of AD only in women (243% greater than men) (Fig. 4). Confirmatory analyses accounting for global cognitive status practically reproduced the aforementioned findings, suggesting that the prognostic properties of the aforementioned NPS are independent of global cognition (Supplementary Table 1).
MCI participant characteristics and missing data
Of the 44,713 participants of the UDS, a total of 6369 with MCI were eligible for the present analysis (Fig. 1). A total of 1773 were not included in any analysis due to missing data on covariates. An additional 151 were not involved in any model because of missing data on all NPS. Among the remaining 4445 participants, 0–4 individuals were excluded from each survival analysis (due to missing data on the specifically analysed NPS), with the exception of night-time behaviours (21 cases with missing data were excluded).
Individuals with missing data (n = 1,924) were older (76.8 ± 8.4 vs. 75.6 ± 8.1 years), had fewer apoE4 alleles, and were more often women, African American or Asian compared to those without missing data (who were more often men and white). DM, hypertension, dyslipidaemia, alcohol or other substance abuse, were more prevalent among those with missing data (missing data analysis not shown).
Throughout the average follow-up of 3.8 ± 3.0 years (range 0.4–15.5 years), 1467 older adults with MCI progressed to AD while 2978 did not. Baseline differences between those who did and did not develop AD are in Table 3.
NPS and incident AD in men and women with MCI
NPS were similarly associated with incident AD in men and women (Table 4). Moderate/severe apathy, in specific, was related to ~ 1.90 greater hazard of progressing to AD in both sexes; therefore, its hazard conferring properties in men were substantially attenuated compared to the CU set. In general, NPS conferred a lesser hazard to individuals with MCI in comparison with CU people, as reflected on the effect size of the associations. Confirmatory analyses accounting for global cognition reproduced these findings (Supplementary Table 2).
Discussion
The present study revealed that apathy, depression and agitation are differentially associated with incident AD in CU men and women. Moderate-severe apathy was the strongest predictor of future AD in CU men but conferred a lesser hazard of incident AD in CU women. Mild depression and agitation increased the hazard of conversion to AD in CU women but not men, while moderate to severe depression was linked to an elevated hazard of progression to AD in both sexes. However, moderate-severe depression was linked to a markedly elevated risk of future AD in CU men compared to women. On the other hand, NPS were similarly associated with future AD in men and women with MCI. Intriguingly, as with previous research, NPS conferred a greater risk to CU individuals rather than to people with MCI, as reflected on the effect size of the estimated associations. Of note, these estimates were underpowered in the investigation of psychotic symptoms (they had the lowest prevalence among all NPS, which is reflected on the precision of our estimates).
Apathy is conceptualized as the lack of motivation and goal-pursuing behaviours while emotional flattening and indifference usually coexist [32]. The experience of negative rather than blunted affect distinguishes depression from apathy [33]. These neuropsychiatric manifestations constitute the most common NPS in people with MCI and AD [1, 34]. Both depressive symptoms and apathy have been consistently associated with an increased risk of incident AD in populations with MCI or intact cognition [5, 11, 14, 35]. However, no published study has investigated sex interactions. Regarding apathy, our findings appear to be in line with its greater burden in men compared to women with AD [10]. Considering the low prevalence of apathy in the general, CU population, it is possible that the identification of moderate-severe symptoms may yield a relatively strong positive prognostic value in the detection of CU male individuals at high-risk of developing AD [36].
Moderate-severe depression was also a moderate to strong predictor of incident AD in CU men. Given, however, the higher prevalence of the disorder in older populations, moderate-severe depressive symptoms should raise physicians’ vigilance for future AD despite a lesser transition hazard than apathy in CU men [37, 38]. On the other hand, depression only conferred a low hazard for AD in women. Based on the well-established female preponderance of this highly-prevalent condition in later life, depressive symptoms may not be major predictors of AD in CU women [37, 38].
Conversely, delusions and hallucinations are very uncommon in the CU, general population and slightly more prevalent in individuals with MCI [39, 40]. It is argued that psychotic symptoms constitute the strongest NPS precursors of dementia, especially in CU older adults [5, 12]. Of note, delusions and hallucinations have stronger affinity to dementia with Lewy bodies, frontotemporal and vascular dementia, compared to AD [5]. Therefore, despite their well-established low sensitivity for AD, late-life onset psychosis is likely a harbinger of all-cause dementia. Close surveillance is warranted when late-life delusions or hallucinations present in either men or women.
Lability symptoms (agitation, irritability, disinhibition, elation, motor disturbances) and appetite disorders, on the other hand, are much more common than psychosis in individuals with intact cognition or MCI [39] and account for a significant portion of caregiver burden [41]. The relationship between these manifestations and incident dementia is less prominent with the published literature suggesting a predominant link to later frontotemporal dementia, and to a lesser extent AD [5, 35]. Apart from mild agitation, this association with AD is sex-independent, and the presence of lability symptoms and appetite disorders should alert physicians to a greater hazard of future AD in both men and women with intact cognition or MCI.
As for sleep disturbances, there is a vast literature confirming their association with future AD, using more thorough assessment protocols than the NPI-Q, separately and meticulously addressing the different aspects of sleep [42]. Of note, sleep disorders are associated with future risk of all-cause dementia and cognitive decline without specific sex-dependent associations known to date.
Strengths and limitations
The main strengths of our study are the large sample size, the long follow-up and the large number of documented events (incident AD). We were careful to exclude individuals with pre-existing psychiatric disorders that may interfere with cognition, as well as to account for important confounders.
This analysis has several weaknesses, as well. First, the diagnosis of AD and other dementias was established by either the examining physician or by an expert-consensus team, based on comprehensive neurological and neuropsychological evaluations (imaging and biological biomarkers were not uniformly available). Although, the exhaustive assessments of the UDS improve the accurate diagnostic characterization of the participants, the presence of misclassification bias cannot be ruled out, especially for cases of mixed dementia. Second, the prevalence of psychosis was low, especially among CU individuals. Therefore, some analyses were underpowered. Third, NPS were assessed using the NPI-Q: while this is a widely used instrument in dementia research, more rigorous assessment tools (e.g., NPI-C- clinician inventory) would be more sensitive in revealing, and more accurate in quantifying, NPS severity. Therefore, more thorough assessment protocols might capture additional associations. Moreover, although we adjusted analyses for several factors, our findings may have been driven by residual confounding (it would not be possible to capture the effect of every potential confounder [43, 44]) or the non-trivial proportion of missing data. Another limitation of this study is its observational nature. In specific, it is not possible to make etiologic inferences about NPS and incident AD, considering that preclinical neurodegenerative brain changes precede the identification of AD for many years. Finally, the current report focused exclusively on AD; therefore, future research ought to investigate sex differences in other dementia entities, as well.
Conclusions
We found that apathy, depression, and agitation are differentially associated with incident AD in CU men versus women. No NPS were related to different risks of progression to AD in older adults with MCI. Of interest, as with previous research, NPS conferred a greater risk to CU individuals rather than to people with MCI, as reflected on the effect size of the associations. These findings may have implications in the early identification of people at high risk to develop AD.
Availability of data and materials
For further information on access to the NACC database, please contact NACC (contact details can be found at https://naccdata.org/).
References
Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288(12):1475. https://doi.org/10.1001/jama.288.12.1475
Zhao Q-F, Tan L, Wang H-F, Jiang T, Tan M-S, Tan L et al (2016) The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord 190:264–271. https://doi.org/10.1016/j.jad.2015.09.069
Krell-Roesch J, Syrjanen JA, Machulda MM, Christianson TJ, Kremers WK, Mielke MM et al (2021) Neuropsychiatric symptoms and the outcome of cognitive trajectories in older adults free of dementia: the Mayo Clinic Study of Aging. Int J Geriatr Psychiatry 36(9):1362–1369. https://doi.org/10.1002/gps.5528
Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJH, Pankratz VS et al (2014) Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry 171(5):572–581. https://doi.org/10.1176/appi.ajp.2014.13060821
Liew TM (2020) Neuropsychiatric symptoms in cognitively normal older persons, and the association with Alzheimer’s and non-Alzheimer’s dementia. Alzheimer’s Res Ther 12(1):35. https://doi.org/10.1186/s13195-020-00604-7
Teng E, Lu PH, Cummings JL (2007) Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement Geriatr Cogn Disord 24(4):253–259. https://doi.org/10.1159/000107100
Defrancesco M, Marksteiner J, Kemmler G, Dal-Bianco P, Ransmayr G, Benke T et al (2020) Specific neuropsychiatric symptoms are associated with faster progression in alzheimer’s disease: results of the Prospective Dementia Registry (PRODEM-Austria). J Alzheimer’s Dis 73(1):125–133. https://doi.org/10.3233/JAD-190662
Rosende-Roca M, Abdelnour C, Esteban E, Tartari JP, Alarcon E, Martínez-Atienza J et al (2021) The role of sex and gender in the selection of Alzheimer patients for clinical trial pre-screening. Alzheimer’s Res Ther 13(1):95. https://doi.org/10.1186/s13195-021-00833-4
Tao Y, Peters ME, Drye LT, Devanand DP, Mintzer JE, Pollock BG et al (2018) Sex differences in the neuropsychiatric symptoms of patients with Alzheimer’s disease. Am J Alzheimer’s Dis Other Dement 33(7):450–457. https://doi.org/10.1177/1533317518783278
Eikelboom WS, Pan M, Ossenkoppele R, Coesmans M, Gatchel JR, Ismail Z et al (2022) Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: a meta-analysis. Alzheimer’s Res Ther 14(1):48. https://doi.org/10.1186/s13195-022-00991-z
Fan Z, Wang L, Zhang H, Lv X, Tu L, Zhang M et al (2021) Apathy as a risky neuropsychiatric syndrome of progression from normal aging to mild cognitive impairment and dementia: a systematic review and meta-analysis. Front Psychiatry 12:792168. https://doi.org/10.3389/fpsyt.2021.792168
Köhler S, Allardyce J, Verhey FRJ, McKeith IG, Matthews F, Brayne C et al (2013) Cognitive decline and dementia risk in older adults with psychotic symptoms: a prospective cohort study. Am J Geriatr Psychiatry 21(2):119–128. https://doi.org/10.1016/j.jagp.2012.10.010
Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C et al (2010) Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimer’s Dis 20(1):175–183. https://doi.org/10.3233/JAD-2010-1352
Roberto N, Portella MJ, Marquié M, Alegret M, Hernández I, Mauleón A et al (2021) Neuropsychiatric profiles and conversion to dementia in mild cognitive impairment, a latent class analysis. Sci Rep 11(1):6448. https://doi.org/10.1038/s41598-021-83126-y
Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Alzheimer’s Disease Centers NIA et al (2007) The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord 21(3):249–258. https://doi.org/10.1097/WAD.0b013e318142774e
Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S et al (2006) The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord 20(4):210–216. https://doi.org/10.1097/01.wad.0000213865.09806.92
Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H et al (2009) The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 23(2):91–101. https://doi.org/10.1097/WAD.0b013e318191c7dd
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, for the Consortium on DLB et al (2005) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology 65(12):1863–1872. https://doi.org/10.1212/01.wnl.0000187889.17253.b1
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7):939–939. https://doi.org/10.1212/WNL.34.7.939
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51(6):1546–1554. https://doi.org/10.1212/WNL.51.6.1546
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56(3):303. https://doi.org/10.1001/archneur.56.3.303
Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH et al (1993) Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 43(2):250–250. https://doi.org/10.1212/WNL.43.2.250
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O et al (2004) Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256(3):240–246. https://doi.org/10.1111/j.1365-2796.2004.01380.x
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44(12):2308–2308. https://doi.org/10.1212/WNL.44.12.2308
Liampas I, Siokas V, Lyketsos CG, Dardiotis E (2022) The relationship between neuropsychiatric symptoms and cognitive performance in older adults with normal cognition. Medicina 58(11):1586. https://doi.org/10.3390/medicina58111586
Liampas I, Siokas V, Ntanasi E, Kosmidis MH, Yannakoulia M, Sakka P et al (2022) Cognitive trajectories preluding the imminent onset of Alzheimer’s disease dementia in individuals with normal cognition: results from the HELIAD cohort. Aging Clin Exp Res. https://doi.org/10.1007/s40520-022-02265-y
Liampas I, Folia V, Zoupa E, Siokas V, Yannakoulia M, Sakka P et al (2022) Qualitative verbal fluency components as prognostic factors for developing Alzheimer’s dementia and mild cognitive impairment: results from the population-based HELIAD Cohort. Medicina 58:1814. https://doi.org/10.3390/medicina58121814
Folia V, Liampas I, Siokas V, Silva S, Ntanasi E, Yannakoulia M et al (2022) Language performance as a prognostic factor for developing Alzheimer’s clinical syndrome and mild cognitive impairment: results from the population-based HELIAD cohort. J Int Neuropsychol Soc. https://doi.org/10.1017/S1355617722000376
Monsell SE, Dodge HH, Zhou XH, Bu Y, Besser LM, Mock C et al (2016) Neuropsychology Work Group Advisory to the Clinical Task Force. Results from the NACC Uniform Data Set Neuropsychological Battery Crosswalk Study. Alzheimer Dis Assoc Disord 30(2):134–139. https://doi.org/10.1097/WAD.0000000000000111
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x. (Erratum in: J Am Geriatr Soc. 2019 Sep;67(9):1991)
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
Fahed M, Steffens DC (2021) Apathy: neurobiology, assessment and treatment. Clin Psychopharmacol Neurosci 19(2):181–189. https://doi.org/10.9758/cpn.2021.19.2.181
Eysenck MW, Fajkowska M (2018) Anxiety and depression: Toward overlapping and distinctive features. Cogn Emot 32(7):1391–1400. https://doi.org/10.1080/02699931.2017.1330255
Martin E, Velayudhan L (2020) Neuropsychiatric symptoms in mild cognitive impairment: a literature review. Dement Geriatr Cogn Disord 49(2):146–155. https://doi.org/10.1159/000507078
Liew TM (2019) Symptom clusters of neuropsychiatric symptoms in mild cognitive impairment and their comparative risks of dementia: a cohort study of 8530 older persons. J Am Med Dir Assoc 20(8):1054.e1-1054.e9. https://doi.org/10.1016/j.jamda.2019.02.012
Sherman C, Liu CS, Herrmann N, Lanctôt KL (2018) Prevalence, neurobiology, and treatments for apathy in prodromal dementia. Int Psychogeriatr 30(2):177–184. https://doi.org/10.1017/S1041610217000527
Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML (2010) High occurrence of mood and anxiety disorders among older adults: the National Comorbidity Survey Replication. Arch Gen Psychiatry 67(5):489. https://doi.org/10.1001/archgenpsychiatry.2010.35
Villarroel MA, Terlizzi EP (2020) Symptoms of depression among adults: United States, 2019. NCHS Data Brief 379:1–8
Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJH, Pankratz VS et al (2008) Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 65(10):1193. https://doi.org/10.1001/archpsyc.65.10.1193
Lyketsos CG (2000) Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry 157(5):708–714. https://doi.org/10.1176/appi.ajp.157.5.708
Keszycki RM, Fisher DW, Dong H (2019) The hyperactivity–impulsivity–irritiability–disinhibition–aggression–agitation domain in Alzheimer’s disease: current management and future directions. Front Pharmacol 10:1109. https://doi.org/10.3389/fphar.2019.01109
Xu W, Tan C-C, Zou J-J, Cao X-P, Tan L (2020) Sleep problems and risk of all-cause cognitive decline or dementia: An updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 91(3):236–244. https://doi.org/10.1136/jnnp-2019-321896
Siokas V, Liampas I, Lyketsos CG, Dardiotis E (2022) Association between motor signs and cognitive performance in cognitively unimpaired older adults: a cross-sectional study using the NACC Database. Brain Sci 12(10):1365. https://doi.org/10.3390/brainsci12101365
Liampas I, Hatzimanolis A, Siokas V, Yannakoulia M, Kosmidis MH, Sakka P et al (2022) Antihypertensive medication class and the risk of dementia and cognitive decline in older adults: a secondary analysis of the prospective HELIAD Cohort. J Alzheimers Dis 89(2):709–719. https://doi.org/10.3233/JAD-220439
Acknowledgements
The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI David Bennett, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), P30 AG072959 (PI James Leverenz, MD).
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
IL: original draft preparation, data curation, formal analysis, design of the study, interpretation of data, and review & editing of manuscript; VS: data curation, validation, review & editing of manuscript; CGL: conceptualization, formulation of research question, design of the study, supervision, review & editing; ED: conceptualization, formulation of research question, design of the study, supervision; review & editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
Participants or surrogates provide informed consent before participation. All procedures are overseen by Institutional Review Boards at each ADRC and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liampas, I., Siokas, V., Lyketsos, C.G. et al. Associations between neuropsychiatric symptoms and incident Alzheimer’s dementia in men versus women. J Neurol 270, 2069–2083 (2023). https://doi.org/10.1007/s00415-022-11541-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11541-w