Abstract
Introduction
Adenosine 2A (A2A) receptors co-localize with dopamine D2 receptors in striatopallidal medium spiny neurons of the indirect pathway. A2A receptor activation in the striatum or pallidum decreases D2 signaling. In contrast, A2A receptor antagonism may help potentiate it. Furthermore, previous PET studies have shown increased A2A receptor availability in striatum of late-stage PD patients with dyskinesia. However, human in vivo evidence for striatal A2A receptor availability in early-stage PD is limited. This study aimed to investigate possible differences in A2A receptor availability in the striatum and pallidum of early- and moderate-stage PD patients without dyskinesias.
Methods
Brain MRI and PET with [11C]TMSX radioligand, targeting A2A receptors, was performed in 9 patients with early- and 9 with moderate-stage PD without dyskinesia and in 6 healthy controls. Distribution volume ratios (DVR) were calculated to assess specific [11C]TMSX binding in caudate, putamen and pallidum.
Results
A2A receptor availability (DVR) was decreased in the bilateral caudate of early-stage PD patients when compared with healthy controls (P = 0.02). Conversely, DVR was increased bilaterally in the pallidum of moderate-stage PD patients compared to healthy controls (P = 0.03). Increased mean striatal DVR correlated with higher motor symptom severity (\(rho\) = 0.47, P = 0.02).
Conclusion
Our results imply regional and disease stage-dependent changes in A2A receptor signaling in PD pathophysiology and in response to dopaminergic medication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a multifaceted neurologic disorder characterized by degeneration of dopaminergic neurons in substantia nigra pars compacta (SNc). This decreases striatal dopamine, resulting in hyperactivity of the GABAergic medium spiny neurons (MSN) of the indirect pathway. It also causes an imbalance of striatal output from both the direct and indirect pathways. This functional imbalance causes motor symptoms in PD [1].
Adenosine 2A (A2A) receptors co-localize with dopamine D2 receptors at the somato-dendritic and nerve terminal level of striatopallidal MSNs of the indirect pathway [2, 3]. They have a homogenous distribution throughout the striatum and are also expressed in the external segment of globus pallidus [2, 4]. Activation of A2A receptors at the dendritic level in the striatum or at the nerve terminal level in globus pallidus externa leads to a decrease in dopaminergic D2 signaling via antagonistic allosteric interaction between these two receptors [5, 6]. Clinical relevance of the interaction between A2A and D2 receptors in PD is best established in advanced stage of the disease, where it forms the basis of the use of A2A antagonists as an add-on medication with levodopa/carbidopa to reduce motor complications, especially the duration of ‘off’ periods in US and Japan [7]. PD patients also exhibit non-motor symptoms such as cognitive deficits and mood-related alterations already early in the disease [8, 9]. Pre-clinical studies based on animal PD models suggest an improvement in working memory impairments and depression like behavior following A2A receptor blockade [10, 11]. A2A antagonists have been shown to decrease cellular death resulting from alpha-synuclein toxicity [12]. It has also been postulated that the effect of A2A antagonists on motor symptoms in early stages of PD may be stronger as compared to advanced stages because of the higher number of viable dopaminergic neurons [13].
Imaging the availability of A2A receptors in vivo in humans is possible using positron emission tomography (PET). However, only a few studies of A2A receptor PET imaging in PD have been reported to date [14,15,16]. These studies have provided preliminary evidence, indicating that the availability of A2A receptors in caudate and putamen is increased in late-stage PD patients with dyskinesia compared to PD patients without dyskinesia and to healthy controls [14, 15]. Also, one of these studies showed an increase in putaminal A2A receptor availability after the initiation of dopaminergic medication in drug-naïve PD patients [14]. Finally, despite the involvement of pallidum in PD pathophysiology, pallidal A2A receptor availability has not been reported with PET at any stage of the disease [17]. We, therefore, sought to explore A2A receptor availability in relevant brain regions of early- and moderate-stage PD patients and compared to healthy controls using PET imaging and [11C]TMSX radioligand which binds selectively to A2A receptors [3].
Methods
Study protocols and patient consent
The protocol for this academic, investigator-initiated, cross-sectional study was approved by the Ethics Committee of the Hospital District of Southwest Finland. All study participants provided signed informed consent and the study was conducted in accordance with the World Medical Association’s declaration of Helsinki.
Study participants
Ten patients with early stage, ten with moderate-stage idiopathic PD and six healthy controls were enrolled for this study. All patients fulfilled the clinically defined criteria for idiopathic Parkinson’s Disease without dyskinesia [18]. PD was considered to be at early stage if time from diagnosis was < 5 years, and at moderate stage if time from diagnosis was between 5 and 15 years. PD cohorts were recruited from the outpatient neurology clinics of Turku University Hospital and through study advertisements published on the Finnish Parkinson Foundation forums. Exclusion criteria included the presence of drug-induced dyskinesia, history of other neurological or psychiatric diseases, or another significant comorbidity. Demographics and clinical features of PD patients and healthy controls are summarized in Table 1. To avoid the possible short-term effects of dopaminergic medications on A2A receptor availability, PET scans for PD patients were performed after 12 h (for standard levodopa) and 24 h (for prolonged release levodopa/carbidopa, MAO-B inhibitors and/or dopamine agonists) cessation of dopaminergic medication (Supplementary Table 1). Moreover, all study subjects were instructed to avoid consuming caffeinated beverages within 24 h before the scanning considering the antagonistic action of caffeine on A2A receptors and half-life of caffeine of 2.3–9.9 h [19,20,21].
Clinical assessment
All patients underwent a thorough medical and neurological examination prior to the PET scan. Definition of the disease duration, Unified Parkinson’s disease rating scale (UPDRS) I–V scoring, Mini Mental Status Examination (MMSE) and calculation of the Levodopa Equivalent Dose (LED) were acquired as part of the examination.
[11C]TMSX radioligand production and administration
Production of [11C]TMSX radioligand was performed according to the methodology described in detail in our earlier study [22]. The radiochemical purity of the produced radioligand was 97.6 (± 0.5) percent. At the start of the dynamic PET scan, a smooth single bolus of [11C]TMSX was injected into the left antecubital vein and was subsequently flushed with saline. The mean (± SD) injected dose (MBq) of [11C]TMSX for controls, and for early-stage and moderate-stage PD patients was 490 (± 25.1), 483 (± 26.6) and 492 (± 21.0), respectively, without significant differences in doses between the groups (P = 0.705). One patient from the early PD group and one from moderate PD group were excluded from the final analyses, since the [11C]TMSX PET data were not reliably quantifiable due to the relatively low injected dose of the radiotracer and high injected mass, respectively.
PET and MR acquisition and image processing
A 60-min dynamic brain PET scan with [11C]TMSX radiotracer was acquired using the ECAT HRRT scanner (Knoxville, USA). Tissue attenuation maps were obtained with a 6-min transmission scan for attenuation correction using 137Cs point source prior to the dynamic scan. Twenty-seven timed frames (6 × 10, 1 × 30, 5 × 60, 5 × 150, and 8 × 300 s) lasting a total of 3600 s were used for PET image reconstruction in accordance with methods described in our earlier study [22].
All subjects underwent brain MRI with 1.5T Nova Dual scanner (Philips, Netherlands). Axial 3D T1 weighted images were used as anatomic reference for PET images. The T1 images were co-registered with motion corrected dynamic PET scans, where the image processing was carried out using Statistical Parametric Mapping software (SPM12, The Wellcome Centre for Human Neuroimaging, UCL, London) as detailed in our earlier study [23]. For the evaluation of region-specific radioligand binding, reference tissue input Logan graphical analysis within 20–60-min interval was used to estimate distribution volume ratios (DVR). This was applied to region of interest (ROI)-based time-activity curves (TAC) using clustered cerebral grey matter as a reference region acquired with a supervised clustering algorithm (modified from the Super PK software) [24]. The supervised clustering algorithm assumes that each dynamic PET data TAC is a combination of four kinetic class TACs that correspond to healthy grey matter, white matter, blood and high specific binding gray matter. In this study, kinetic class TACs corresponding to normal [11C]TMSX binding in grey matter, white matter and blood were first defined from a healthy volunteer group (n = 7). The kinetic class TAC corresponding to high specific [11C]TMSX binding was acquired from thalamus in multiple sclerosis patients (n = 12) and was compared with anterior putamen binding in PD patients (n = 9), as described in [23]. Next, the contribution of each kinetic class to the voxel-level [11C]TMSX TACs was estimated by using non-negative least squares estimation. Lastly, the clustered reference region TAC was calculated from the dynamic [11C]TMSX PET data as a weighted average by using the grey matter coefficients as weighting factors. Parametric maps for voxel-level analysis were generated using non-displaceable binding potential (BPND) images using basis function implementation of simplified reference tissue model (SRTM) with 250 basis functions, and by using the values 0.06 and 0.8 for the lower and upper limit for θ3.
Region of interest and voxel-level analyses of the [11C]TMSX PET data
For the ROI analyses, Freesurfer software (v6.0, http://surfer.nmr.mgh.harvard.edu/) was used for anatomical parcellation of the 3D T1 MR images to extract region-specific DVR values from the co-registered PET scans [25]. Analyses were limited to three bilateral ROIs, i.e., caudate, putamen and pallidum for the primary outcome.
Mean DVR values of the whole dorsal striatum were calculated using a combined mask of caudate and putamen, to check associations with clinical parameters following previous studies [15]. To assess the within and between-group effect of lateralization, contralateral and ipsilateral ROIs were drawn manually for caudate and putamen using in-house software (Carimas v2.9). These unilateral ROIs were defined as contralateral or ipsilateral with respect to each patient’s clinically more affected side, based on the respective unilateral sums of the UPDRS III scores. Comparison of DVRs in ipsilateral or contralateral ROIs of the PD groups with healthy controls was carried out against the combined average DVR of the left and right striatal ROIs of controls.
Parametric [11C]TMSX BPND images were used to evaluate voxel-wise differences between healthy controls and patients with early- and moderate-stage PD utilizing SPM12. Images were smoothed with 3D Gaussian 4 mm FWHM filter to improve signal-to-noise ratio and ensure normal data-distribution before voxel-wise analyses.
Statistical methods
ROI-level statistical analyses were performed with GraphPad Prism software (v9.1, California, USA). Normality of distribution was checked graphically and using the Shapiro–Wilk test. Differences in categorical variables were evaluated using non-parametric Fischer’s exact test. Mann–Whitney U test was used to assess differences in disease duration between patients with early- vs. moderate-stage PD. One-way ANOVA was utilized to evaluate differences in age, molar activity, injected mass, radiochemical purity and injected radioligand dose between groups. To investigate local changes at the ROI level, an independent t-test was used with Bonferroni correction for multiple comparisons applied afterward. Paired t-test was utilized to calculate the within-group differences of ipsilateral and contralateral sides determined by the clinically more affected side of the patient. Cohen’s d was calculated to measure the effect size for statistically significant comparisons. Spearman test was used to evaluate correlations between clinical parameters and striatal and pallidal [11C]TMSX DVR values. P-values < 0.05 from two-tailed tests were considered statistically significant in ROI-level analyses.
For the voxel-level analyses in SPM12, a two-sample t-test was carried out using parametric BPND image with overlaid striatal mask, including pallidum, in MNI-152 space. The cluster-based inference with cluster-defining threshold of P = 0.001 (T = 3.85) was used, and the P < 0.05 family-wise error-corrected critical cluster size was 115 voxels.
Results
No differences were observed in the age (P = 0.336) and sex distribution (P = 0.318) between healthy control and early- and moderate-stage PD groups. Patients with moderate-stage PD had a longer disease duration (P < 0.001), higher UPDRS III score (P < 0.001) and LED (P < 0.001) compared to the early-stage PD patients (Table 1).
ROI-based analyses
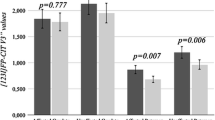
ROI-level analyses revealed that patients with early-stage PD had lower mean [11C]TMSX DVR in bilateral caudate when compared to healthy controls (effect size [ES] = 1.64, P = 0.02) (Fig. 1a, b). No differences were observed in the bilateral caudate DVRs of early-stage patients when compared to moderate-stage patients (P = 0.14), and in the bilateral caudate DVRs of the patients with moderate stage when compared to healthy controls (P > 0.99). Conversely, an increase in mean [11C]TMSX DVR in bilateral pallidum was observed in moderate-stage PD when compared with healthy controls (ES = 1.64, P = 0.03), whereas no difference was observed in the bilateral pallidum when comparing early-stage PD group with moderate-stage PD (P > 0.99) and healthy controls (P = 0.64) (Fig. 2). No significant differences were observed in bilateral putamen DVRs in the group comparisons of early-stage PD vs. controls P > 0.99, moderate-stage PD vs. controls (P = 0.74) and early-stage PD vs. moderate-stage PD (P = 0.42) (Fig. 1a).
a Boxplot with distribution volume ratio (DVR) values representing [11C]TMSX binding in adenosine A2A receptors in the striatum of healthy control (HC), Parkinson's disease early-stage (PDE) and Parkinson's disease moderate-stage (PDM) groups. The availability of A2A receptors is decreased in bilateral caudate nucleus in PDE (P = 0.02, Independent t-test) when compared to HC. A2A receptor availability in bilateral pallidum in PDM is increased when compared to HC (P = 0.03, Independent t-test). *P < 0.05 was considered statistically significant after correction for multiple comparison. b Mean parametric DVR image of HC, PDE and PDM patients overlaid on MNI 152 template demonstrating A2A receptor availability in the striatum with the binding in the head of caudate nucleus enlarged. c Voxel-level analyses depicting a decrease in BPND in clusters defining bilateral caudate (P < 0.001) of early PDE patients when compared with HC. Decreases can also be observed in left (and to a lesser extent right) anterior putamen of PDE patients vs HCs. The cluster-based inference with cluster-defining threshold of P = 0.001 (T = 3.85) was used and the P < 0.05 family-wise error-corrected critical cluster size was 115
Mean parametric DVR pallidum mask of healthy controls (HC), Parkinson's disease early-stage (PDE) and Parkinson's disease moderate-stage (PDM) patients overlaid on ch2better template demonstrating higher A2A receptor availability in PDM as compared to HC (P = 0.03, Independent t-test). P < 0.05 was considered statistically significant after correction for multiple comparisons
In the evaluation of the unilateral striatal ROIs in relation to the clinically more affected (contralateral) and less affected (ipsilateral) sides among the PD patients, significant decrease was observed in the ipsilateral caudate of early-stage PD patients (ES = 1.76, P = 0.01) compared to the average caudate DVR of healthy controls. Similar, but non-significant trend was observed for the comparison of contralateral caudate of early-stage PD to controls, (ES = 1.38, P = 0.06). No difference was observed in the ipsilateral vs. contralateral within-group comparison of caudate in the early-stage PD patients (Table 2b). Similarly, an increase in the uptake of [11C]TMSX in ipsilateral pallidum of moderate-stage PD patients when compared to healthy controls was noted (ES = 1.83, P = 0.02) but not for the contralateral pallidum (P = 0.92). Again, no difference was observed in the ipsilateral vs. contralateral region of interest comparison within the PD moderate group (Table 2b).
The mean striatal [11C]TMSX DVR correlated with UPDRS III scores (\(rho\) = 0.47, P = 0.02) within the pooled group of PD patients (Fig. 3a). However, no correlations were observed between the mean DVRs separately in caudate, putamen, pallidum and the UPDRS III scores (Table 3). On the other hand, we noticed a moderate association between [11C]TMSX binding in striatum contralateral to the side with predominant motor symptoms and the UPDRS III score of the clinically less affected side (\(rho\) = 0.47, P = 0.04) and a similar but non-significant trend with the UPDRS III score of the clinically more affected side (\(rho\) = 0.42, P = 0.08) in the pooled PD group (Fig. 3b, 3c). Moderate correlation was also observed between [11C]TMSX DVR in the striatum ipsilateral to the side with predominant motor symptoms and the UPDRS III score of the clinically more affected side of the body (\(rho\) = 0.48, P = 0.04) as well as with the UPDRS III score of the clinically less affected side (\(rho\) = 0.49, P = 0.04) in pooled group of PD patients (Fig. 3d, e).
Scatter plots showing associations between [11C]TMSX distribution volume ratio (DVR) representing adenosine A2A receptor availability in whole, ipsilateral (Ipsi) and contralateral (Contra; relative to the clinically more affected side) striatum and UPDRS III scores of pooled Parkinson’s disease (PD) patients (PD early + PD moderate). a Correlation between mean bilateral striatum DVR of pooled PD patients and their total UPDRS III score. b Correlation between mean contralateral striatum DVR of pooled PD patients and the UPDRS III score of their clinically more affected side. c Correlation between mean contralateral striatum DVR of pooled PD patients and the UPDRS III score of their clinically less affected side. d Correlation between mean ipsilateral striatum DVR of pooled PD patients and the UPDRS III score of their clinically more affected side. e Correlation between mean ipsilateral striatum DVR of pooled PD patients and the UPDRS III score of their clinically less affected side. UPDRS: Unified Parkinson’s disease rating scale
We also observed an association between striatal [11C]TMSX binding and levodopa equivalent dose (LED) in patients with early-stage PD (\(rho\) = – 0.68, P = 0.02) (Fig. 4a). Mean bilateral caudate [11C]TMSX DVR also correlated with LED in pooled PD patients (\(rho\) = 0.55, P = 0.02) (Fig. 4b). Similarly, a strong correlation was found between [11C]TMSX binding in bilateral putamen and LED (\(rho\) = – 0.76, P = 0.02) as well as with disease duration (\(rho\) = – 0.82, P = 0.01) in early-stage PD patients (Table 3, Fig. 4c and 4d).
Scatter plots showing associations between [11C]TMSX distribution volume ratio (DVR) representing adenosine A2A receptor availability in striatal regions of interest and clinical parameters of Parkinson’s disease (PD) patients. a Correlation between mean bilateral striatum DVR of PD early-stage (PDE) patients and their Levodopa equivalent dose (LED). b Correlation between mean bilateral caudate DVR of pooled PD patients (PD early-stage + PD moderate-stage patients) and their LED. c Correlation between mean bilateral putamen DVR of PDE patients and their LED. d Correlation between mean bilateral putamen DVR of PDE patients and their disease duration
Voxel-level analyses
Voxel-level analyses showed decreased BPND in clusters defining bilateral caudate (P < 0.001) of early-stage PD patients when compared with healthy controls (Fig. 1c). Similarly, decreased BPND was also observed in left anterior putamen, and to a lesser extent in right anterior putamen of early-stage PD subjects when compared to controls. However, no differences were observed when comparing moderate-stage PD patients with healthy controls and early-stage PD patients (data not shown).
Discussion
Our work demonstrates that A2A receptor availability is significantly decreased in the caudate in early-stage PD patients when compared to healthy controls. Furthermore, an increased availability of adenosine A2A receptors in the pallidum in moderate-stage PD patients was observed compared to healthy controls.
A decrease in A2A receptor availability in the bilateral caudate of early-stage PD patients was evident both in the region of interest as well as the voxel-level analyses (Fig. 1). This is in line with evidence from pre-clinical and post-mortem PD studies, suggesting a decrease of A2A receptors in the caudate nucleus but not in the putamen [26,27,28]. Interestingly, [11C]Raclopride studies targeting dopamine D2 receptors, which co-localize with A2A receptors in striatum, have also shown a decrease in binding in caudate of early-stage PD patients while binding in the putamen was similar to healthy volunteers [29, 30]. Experimental studies demonstrate negative allosteric modulation of A2A receptor binding by D2 agonists such as pramipexole and rotigotine [31]. Previously, we have shown that unlike in putamen and pallidum, the repeatability of [11C]TMSX binding in caudate and its subregions is poor when comparing DVR values during and after a short-term (12–24 h) cessation of routine dopaminergic medication [32]. Our current results also suggest a correlation between higher LED and higher [11C]TMSX binding in the caudate of pooled PD patients. On the other hand, we have shown an association between higher LED and lower [11C]TMSX binding in striatum and putamen of early-stage patients. Taking all these findings into consideration, we propose that dopaminergic medication affects A2A-D2 heterodimer in caudate differentially as compared to the rest of striatum. This selective reduction could be attributed to the regional differentiation in the subpopulation of A2A receptors or to the relatively heavier reliance on dopamine agonists in earlier stages of the disease (Supp Table 1) [30]. Previous studies suggest that the degeneration of dopaminergic neurons projecting to putamen precedes those projecting to caudate [33]. This difference in dopamine may also explain the decrease in A2A receptor availability in caudate and not putamen of early PD patients. Another possible, but less likely, explanation could be the degeneration of dendritic spines of striatopallidal MSNs, where A2A receptors are heavily expressed, as the disease progresses [34].
We also observed an increase in bilateral pallidal availability of A2A receptors in the moderate stage of the disease at ROI level compared with healthy controls (Fig. 2). However, the result could not be replicated at the voxel-level likely due to the high level of noise in dynamic PET scan TACs and the strict cluster-defining threshold. Within the pallidum, A2A receptors are mainly located on the nerve terminals of GABAergic striatopallidal neurons in the external segment of globus pallidus [2, 4, 35]. A2A receptor-mediated modulation of the indirect pathway output occurs not only at the striatal level but also at the pallidal nerve terminals [36]. However, this ‘dual excitatory modulation’ is relatively less potent in globus pallidus externa as compared to striatal A2A receptors [37]. Our result is in agreement with previous post-mortem evidence where binding of A2A receptor specific ligand was found to be higher in the external segment of globus pallidus of postmortem brains of PD patients compared to controls [27]. Recent evidence suggests an increase in ATP in nigrostriatal pathway and CD73-mediated increase in adenosine within striatum [38, 39]. This extracellular increase in adenosine could explain the compensatory increases in pallidal A2A receptor availability in moderate PD patients in our study and also the increase in striatal A2A receptor binding in PD patients with dyskinesia reported earlier [14, 15]. Increased receptor expression invariably results in higher G protein-coupled receptor (GPCR) activity [40]. This implies that higher pallidal A2A receptor expression could possibly counteract the effect of antiparkinsonian therapeutics on indirect pathway in moderate-stage patients. It is imperative to note that therapeutic modulation of the indirect pathway is more important to reduce motor disability in PD as compared to the direct pathway [1]. Therefore, higher pallidal expression of A2A receptors can be one potential mechanism contributing to the decreased dose-related efficacy of dopaminergic medication in PD patients during disease progression [41]. Increased A2A receptor expression may also alter output of globus pallidus externa to other basal ganglia structures as it innervates the striatum, subthalamic nucleus, globus pallidus interna and substantia nigra pars reticulata [42].
Our data also demonstrate that the decrease in [11C]TMSX binding in caudate of early-stage PD patients and the increase in pallidum of moderate-stage patients is slightly more pronounced on the ipsilateral side with respect to predominant motor symptoms. A significant association was shown between [11C]TMSX binding of bilateral striatum and UPDRS III scores of pooled PD patients. Similarly, we also observed moderate association of ipsilateral striatal [11C]TMSX binding with the UPDRS III score of the more and less affected side. Contrarily, the binding of contralateral striatum had a moderate association with the UPDRS III score of the clinically less affected side only. An earlier [11C]TMSX PET study found an asymmetric decrease in putaminal binding in the more affected side when compared with the less affected side in drug naïve patients [14]. Similarly, an increasing number of PET studies examining dopaminergic degeneration have shown a more pronounced deficit in the contralateral striatum. However, some studies report it to be higher in the ipsilateral striatum relative to the clinically more affected side of the body possibly due to ‘floor effect’[43,44,45]. This factor may also be responsible for the higher change in binding on the ipsilateral side in our study [46].
We also found a strong correlation between lower bilateral putaminal [11C]TMSX binding and higher disease duration in early-stage patients. However, no correlation was found between disease duration and striatal or pallidal binding in moderate-stage patients. This inconsistency could potentially be attributed to compensatory increase in A2A receptor expression in later stages of the disease [14, 15]. Furthermore, since our findings are based on a limited number of patients and we lack the imaging data of the dopaminergic system, the results from our study should be considered preliminary [43]. In addition, the low signal-to-noise ratio of [11C]TMSX should also be considered a potential limitation of our work. Instead of using centrum semiovale as reference region or the ‘gold standard’ arterial blood sampling, we used clustered cerebral grey matter as reference region for our work [47]. This method was validated and found to be as robust as arterial blood sampling for use with [11C]TMSX in our earlier work [23, 32].
Mishina and colleagues[14] found an asymmetric decrease in [11C]TMSX binding in the putamen of clinically more affected side as compared to less affected side in drug naïve patients. The availability of A2A receptors in bilateral putamen increased in the same patients approximately 15 months after starting regular dopaminergic medication [14]. However, they did not report of a comparison to healthy controls in these patients after the period of dopaminergic medication usage, so the inference on the magnitude of longitudinal change remains limited.
In conclusion, we have demonstrated that the availability of A2A receptors is decreased in the caudate in patients with early-stage PD and increased in the pallidum in patients with moderate-stage PD. Further studies are warranted to confirm these findings and to investigate A2A-related pathological changes in PD.
Data statement
The raw data used in the preparation of this article can be shared in anonymized format by request of a qualified investigator.
References
Obeso JA, Marin C, Rodriguez-Oroz C et al (2008) The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol 64:S30–S46. https://doi.org/10.1002/ana.21481
Svenningsson P, Hall H, Sedvall G, Fredholm BB (1997) Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse 27:322–335. https://doi.org/10.1002/(SICI)1098-2396(199712)27:4%3c322::AID-SYN6%3e3.0.CO;2-E
Mishina M, Ishiwata K, Kimura Y et al (2007) Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse 61:778–784. https://doi.org/10.1002/syn.20423
Rosin DL, Hettinger BD, Lee A, Linden J (2003) Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. https://doi.org/10.1212/01.wnl.0000095205.33940.99
Ferré S, Fredholm BB, Morelli M et al (1997) Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20:482–487. https://doi.org/10.1016/S0166-2236(97)01096-5
Díaz-Cabiale Z, Hurd Y, Guidolin D et al (2001) Adenosine A2A agonist CGS 21680 decreases the affinity of dopamine D2 receptors for dopamine in human striatum. NeuroReport 12:1831–1834. https://doi.org/10.1097/00001756-200107030-00014
Chen JF, Cunha RA (2020) The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal 16:167–174
Schrag A (2004) Psychiatric aspects of Parkinson’s disease: an update. J Neurol 251:795–804. https://doi.org/10.1007/s00415-004-0483-3
Chaudhuri KR, Healy DG, Schapira AHV (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245. https://doi.org/10.1016/S1474-4422(06)70373-8
Ko WKD, Camus SM, Li Q et al (2016) An evaluation of istradefylline treatment on Parkinsonian motor and cognitive deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated macaque models. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2016.07.012
Kaster MP, Machado NJ, Silva HB et al (2015) Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A 112:7833–7838. https://doi.org/10.1073/pnas.1423088112
Ferreira DG, Batalha VL, Vicente Miranda H et al (2015) Adenosine A2A receptors modulate α-synuclein aggregation and toxicity. Cereb Cortex N Y N. https://doi.org/10.1093/cercor/bhv268
Fuxe K, Borroto-Escuela DO, Romero-Fernandez W et al (2014) Moonlighting proteins and protein-protein interactions as neurotherapeutic targets in the G protein-coupled receptor field. Neuropsychopharmacology 39:131–155
Mishina M, Ishiwata K, Naganawa M et al (2011) Adenosine A2A receptors measured with [11C]TMSX PET in the striata of Parkinson’s disease patients. PLoS ONE 6:e17338. https://doi.org/10.1371/journal.pone.0017338
Ramlackhansingh AF, Bose SK, Ahmed I et al (2011) Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology 76:1811–1816. https://doi.org/10.1212/WNL.0b013e31821ccce4
Ishibashi K, Miura Y, Wagatsuma K et al (2018) Occupancy of adenosine A2A receptors by istradefylline in patients with Parkinson’s disease using [11C]Preladenant PET. Neuropharmacology 143:106–112. https://doi.org/10.1016/j.neuropharm.2018.09.036
Mastro KJ, Zitelli KT, Willard AM et al (2017) Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat Neurosci 206(20):815–823. https://doi.org/10.1038/nn.4559
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Ferré S, Bonaventura J, Tomasi D et al (2016) Allosteric mechanisms within the Adenosine A2A Dopamine D2 receptor heterotetramer. Neuropharmacology 104:154–160. https://doi.org/10.1016/j.neuropharm.2015.05.028
Ishibashi K, Miura Y, Wagatsuma K et al (2022) Adenosine A2A receptor occupancy by caffeine after coffee intake in Parkinson’s disease. Mov Disord. https://doi.org/10.1002/mds.28897
Nehlig A (2018) Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol Rev 70:384–411. https://doi.org/10.1124/pr.117.014407
Rissanen E, Virta JR, Paavilainen T et al (2013) Adenosine A2A receptors in secondary progressive multiple sclerosis: a [11C]TMSX brain PET study. J Cereb Blood Flow Metab 33:1394–1401. https://doi.org/10.1038/jcbfm.2013.85
Rissanen E, Tuisku J, Luoto P et al (2015) Automated reference region extraction and population-based input function for Brain [11C]TMSX PET image analyses. J Cereb Blood Flow Metab 35:157–165. https://doi.org/10.1038/jcbfm.2014.194
Turkheimer FE, Edison P, Pavese N et al (2007) Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nucl Med 48:158–167
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. https://doi.org/10.1016/S0896-6273(02)00569-X
Hurley MJ, Mash DC, Jenner P (2000) Adenosine A2A receptor mRNA expression in Parkinson’s disease. Neurosci Lett 291:54–58. https://doi.org/10.1016/S0304-3940(00)01371-9
Calon F, Dridi M, Hornykiewicz O et al (2004) Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain 127:1075–1084. https://doi.org/10.1093/brain/awh128
Trincavelli ML, Daniele S, Orlandini E et al (2012) A new D2 dopamine receptor agonist allosterically modulates A2A adenosine receptor signalling by interacting with the A2A/D2 receptor heteromer. Cell Signal 24:951–960. https://doi.org/10.1016/j.cellsig.2011.12.018
Antonini A, Schwarz J, Oertel WH et al (1997) Long-term changes of striatal dopamine D2 receptors in patients with Parkinson’s disease: a study with positron emission tomography and [11C]Raclopride. Mov Disord 12:33–38. https://doi.org/10.1002/mds.870120107
Politis M, Wilson H, Wu K et al (2017) Chronic exposure to dopamine agonists affects the integrity of striatal D2 receptors in Parkinson’s patients. NeuroImage Clin 16:455–460. https://doi.org/10.1016/j.nicl.2017.08.013
Fernández-Dueñas V, Gómez-Soler M, Morató X et al (2013) Dopamine D2 receptor-mediated modulation of adenosine A2A receptor agonist binding within the A2AR-D2R oligomer framework. Neurochem Int 63:42–46. https://doi.org/10.1016/j.neuint.2013.04.006
Waggan I, Rissanen E, Tuisku J et al (2021) Effect of dopaminergic medication on adenosine 2A receptor availability in patients with Parkinson’s disease. Parkinsonism Relat Disord 86:40–44. https://doi.org/10.1016/j.parkreldis.2021.03.030
Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. N Engl J Med 318:876–880. https://doi.org/10.1056/NEJM198804073181402
Day M, Wang Z, Ding J et al (2006) Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9:251–259. https://doi.org/10.1038/nn1632
Bogenpohl JW, Ritter SL, Hall RA, Smith Y (2012) Adenosine A2A receptor in the monkey Basal Ganglia: ultrastructural localization and colocalization with the metabotropic glutamate receptor 5 in the striatum. J Comp Neurol 520:570–589. https://doi.org/10.1002/cne.22751
Ochi M, Koga K, Kurokawa M et al (2000) Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: a microdialysis study. Neuroscience 100:53–62. https://doi.org/10.1016/S0306-4522(00)00250-5
Shindou T, Richardson PJ, Mori A et al (2003) Adenosine modulates the striatal GABAergic inputs to the globus pallidus via adenosine A2A receptors in rats. Neurosci Lett 352:167–170. https://doi.org/10.1016/j.neulet.2003.08.059
Prasad K, de Vries EFJ, Elsinga PH et al (2021) Allosteric interactions between adenosine A2A and dopamine D2 receptors in heteromeric complexes: biochemical and pharmacological characteristics, and opportunities for PET imaging. Int J Mol Sci 22:1719. https://doi.org/10.3390/ijms22041719
Rodrigues RJ, Tomé AR, Cunha RA (2015) ATP as a multi-target danger signal in the brain. Front Neurosci 9:148. https://doi.org/10.3389/fnins.2015.00148
Fredholm BB, Svenningsson P (2020) Why target brain adenosine receptors? A historical perspective. Parkinsonism Relat Disord 80:S3–S6. https://doi.org/10.1016/j.parkreldis.2020.09.027
Lewitt PA (2008) Levodopa for the treatment of Parkinson’s disease. NEJM 359:2468–2476. https://doi.org/10.1056/nejmct0800326
Bergman H (2021) The hidden life of the basal Ganglia: at the base of brain and mind. MIT Press, Cambridge
Roussakis AA, Zeng Z, Lao-Kaim NP et al (2020) Parkinson’s disease laterality: a [11C]PE2I PET imaging study. J Neurol 268:582–589. https://doi.org/10.1007/s00415-020-10204-y
Nandhagopal R, Kuramoto L, Schulzer M et al (2009) Longitudinal progression of sporadic Parkinson’s disease: a multi-tracer positron emission tomography study. Brain 132:2970–2979. https://doi.org/10.1093/brain/awp209
Simuni T, Siderowf A, Lasch S et al (2018) Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s progression markers initiative cohort. Mov Disord 33:771–782. https://doi.org/10.1002/mds.27361
Kaasinen V, Vahlberg T (2017) Striatal dopamine in Parkinson disease: a meta-analysis of imaging studies. Ann Neurol 82:873–882. https://doi.org/10.1002/ana.25103
Naganawa M, Kimura Y, Mishina M et al (2007) Quantification of adenosine A2A receptors in the human brain using [11C]TMSX and positron emission tomography. Eur J Nucl Med Mol Imaging 34:679–687. https://doi.org/10.1007/s00259-006-0294-0
Acknowledgements
We would like to acknowledge our patients and volunteers for making this work possible. We would also like to thank Marjo Nylund, Markus Matilainen, Richard Aarnio and the staff of Turku PET Center for their help and support with realizing this study.
Funding
Open Access funding provided by University of Turku (UTU) including Turku University Central Hospital. This work was supported by Finnish Parkinson Foundation, Finnish Academy and The Hospital District of Southwest Finland State Research Funding. Imran Waggan and Eero Rissanen have been supported by the Finnish Parkinson Foundation. Jouni Tuisku was supported by Finnish Governmental Research Funding (ERVA). Juho Joutsa was supported by the Finnish Medical Foundation, Finnish Parkinson Foundation, Finnish Foundation for Alcohol Studies and Finnish Governmental Research Funding (ERVA); and received a lecturer honorarium from Lundbeck. Juha O. Rinne was supported by the Academy of Finland (project# 310962), the Sigrid Juselius Foundation and Finnish Governmental Research Funding (ERVA). Laura Airas was supported by Finnish Academy, the Sigrid Juselius Foundation and Finnish Governmental Research Funding (ERVA).
Author information
Authors and Affiliations
Contributions
Conceptualization: ER, LA, and JR; methodology: JT, JJ, RP, and SH; formal analysis and investigation: IW; writing—original draft preparation: IW; writing—review and editing: ER, LA, JR, JJ, and SH; funding acquisition: LA; resources: LA; supervision: ER, JR, and LA. All the authors have approved this version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval
The protocol for this academic, investigator-initiated, cross-sectional study was approved by the Ethics Committee of the Hospital District of Southwest Finland. All study participants provided signed informed consent and the study was conducted in accordance with the World Medical Association’s declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waggan, I., Rissanen, E., Tuisku, J. et al. Adenosine A2A receptor availability in patients with early- and moderate-stage Parkinson’s disease. J Neurol 270, 300–310 (2023). https://doi.org/10.1007/s00415-022-11342-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11342-1