Abstract
Aims
We aim to provide real-world evidence on the use of ocrelizumab for treating multiple sclerosis (MS), with specific regard to prescription pattern, adherence, persistence, healthcare resource utilization and related costs, also in relation to other disease-modifying treatments (DMTs).
Methods
We included 2495 people with MS from the Campania Region (South Italy) who received first or switch DMT prescription from Jan 2018 to Dec 2020, and with at least 6-month follow-up. We collected hospital discharge records, drug prescriptions, and related costs, and calculated persistence (time from first prescription to discontinuation or switch to other DMT), adherence (proportion of days covered (PDC)), annualized hospitalization rate (AHR) for MS-related hospital admissions, and DMT costs.
Results
Ocrelizumab was the most commonly prescribed DMT (n = 399; age = 45.74 ± 10.98 years; females = 224), after dimethyl fumarate (n = 588) and fingolimod (n = 401); 26% patients treated with ocrelizumab were naïve. When compared with ocrelizumab, the risk of discontinuation was higher for other highly active DMTs (HR = 3.78; p = 0.01), and low/medium efficacy DMTs (HR = 7.59; p < 0.01). When compared with ocrelizumab, PDC was similar to other highly active DMTs (Coeff = 0.01; p = 0.31), but higher for low/medium efficacy DMTs (Coeff = 0.09; p < 0.01). When compared with ocrelizumab, AHR was similar to other highly active DMTs (Coeff = 0.01; p = 0.51), and low/medium efficacy DMTs (Coeff = 0.01; p = 0.55). When compared with ocrelizumab, DMT monthly costs were higher for other highly active DMTs (Coeff = 92.30; p < 0.01), but lower for low/medium efficacy DMTs (Coeff = − 1043.61; p < 0.01).
Discussion
Ocrelizumab was among the most frequently prescribed DMTs, with 26% prescriptions to treatment-naïve patients, suggesting its relevance in addressing unmet clinical needs (e.g., first approved treatment for primary progressive MS). Ocrelizumab was associated with the highest persistence, confirming its favorable benefit-risk profile. Costs for ocrelizumab were lower than those associated to similarly effective DMTs, in absence of changes in healthcare resource utilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ocrelizumab is approved for the use in both relapsing–remitting and primary progressive multiple sclerosis (MS) [1, 2]. Ocrelizumab efficacy and safety have been preliminarily explored in clinical trials and their long-term extensions [3, 4]. More recently, insights on ocrelizumab real-world use and related clinical efficacy have been gained through clinical registries [5, 6]. However, clinical registries do not include healthcare resource utilization and, more in general, do not cover the complexity of MS management [7, 8]. Also, few studies have directly compared different DMTs in terms of efficacy measures [9]. Datasets based on routinely collected healthcare data can overcome these limitations and provide detailed information on healthcare resource utilization in the long term and on fully representative populations [10]. In the Campania Region of Italy, we have developed an algorithm, specific for individuals with a diagnosis of MS, to merge healthcare data (e.g. planned and unplanned hospital admissions with related diagnoses and costs) and prescription data [11], and to derive measures of DMT utilization (e.g., adherence, persistence) and economic viability.
Hereby, we aim to provide real-world evidence on the use of ocrelizumab, with specific regard to prescription pattern, persistence, adherence, healthcare resource utilization and related costs, and also to compare ocrelizumab to other DMTs, based on administration (e.g., injectable, oral, and infusion) and activity (e.g., low/medium efficacy and highly active DMTs).
Methods
Study design
This is a population-based study, based on the retrospective analysis of routinely collected healthcare data, prospectively recorded from 2018 to 2020, on individuals with a diagnosis of MS living in the Campania Region of Italy. The original dataset has been fully described elsewhere [11]. For the purposes of the present study, we have selected this time frame to include ocrelizumab-treated patients, from the beginning of its use in the real-world (first prescription is recorded on Nov 6, 2018).
The study was approved by the Federico II Ethics Committee (355/19). All patients signed informed consent authorizing the use of anonymized data collected routinely as part of the clinical practice, in line with data protection regulation (GDPR EU2016/679). The study was performed in accordance with good clinical practice and Declaration of Helsinki.
Population
The dataset was created by merging different data sources of the Campania Region [11]. We specifically included all individuals resident in the Campania Region who had at least one MS record, from 2018 to 2020, in the Hospital Discharge Record database, the Regional Drug Prescription database, or the outpatient database with payment exemptions for MS. The case-finding algorithm has 99.0% sensitivity, with very low risk of missing individuals (2.7%) [11]. We have referred to both individual patients and individual treatment periods (ITPs), since the same patient could have been using different DMTs during the study period.
Inclusion criteria were: (1) new DMT prescriptions from Jan 1, 2018, to Dec 31, 2020 (switch from a previous DMT or DMT start in absence of previous treatment records, using data from 2015 to 2017 as characterization period); (2) DMT prescription maintained for at least 6 months (e.g., corresponding to two full infusions for ocrelizumab).
Exclusion criteria were: (1) individual treatment periods already including a DMT at baseline (Jan 1, 2018); (2) incomplete records; (3) lack of written consent to participate in the study; (4) residence outside of the Campania Region.
Treatment variables
DMT prescriptions were collected, and following DMT groups were defined based on:
-
DMT administration route: infusion (alemtuzumab, natalizumab), oral (cladribine, fingolimod, teriflunomide, dimethyl fumarate), and injection (glatiramer acetate, interferon beta-1a, interferon beta-1b, and peg-interferon beta-1a), using ocrelizumab as reference for comparison [12];
-
DMT treatment line: low/medium efficacy (teriflunomide, dimethyl fumarate, glatiramer acetate, interferon beta-1a, interferon beta-1b, and peg-interferon beta-1a) and highly active treatments (alemtuzumab, natalizumab, cladribine, fingolimod), using ocrelizumab as reference for comparison [13, 14].
Based on DMT prescriptions in the previous 12 months, ITPs were classified into treatment naïve (no treatment records in the previous 12 months) and switcher patients (presence of previous treatment records).
Persistence, adherence, healthcare resource utilization and costs
DMT discontinuation was defined as a switch to another DMT or complete discontinuation (i.e., no further record of medication initiation) [8, 13].
Adherence was calculated as the proportion of days covered (PDC) over 1-year time (total days covered during 1 year divided by 365 days of follow-up, using the expected refill/retreatment timing from current regulatory indications); PDC ≥ 0.8 was considered adherent [12]. Considering that some DMTs have low frequency administration that would have caused too much variability in estimating adherence in 6 months (e.g., alemtuzumab, cladribine, ocrelizumab), we have included in adherence analyses only patients with at least 12 months’ follow-up.
Healthcare resource utilization included MS-related and non-MS-related hospital admissions, that were classified based on the main discharge diagnosis. The number of hospital admissions was then reported on annual basis (annualized hospitalization rates (AHR)) [13, 15].
Direct healthcare costs were derived from regional datasets, referred to corresponding healthcare resource utilization, and inflated to the most recent values (2020), to avoid variations in price per unit of service through different years [13, 15].
We further collected age, sex, and, for patients with Hospital Discharge Records, Charlson Comorbidity Index [15, 16].
Statistics
Study variables are presented as mean (± standard deviation), number (percent) or median (range), as appropriate.
Differences between DMT groups (using ocrelizumab as reference in the statistical models) were explored using Cox regression models (i.e., persistence), and linear regression models (i.e., adherence, AHR, costs), as appropriate. Covariates were age, sex, year of treatment start (2018, 2019, or 2020), treatment duration, and adherence; statistical models were then run for the subgroup of patients with hospital discharge records, also including Charlson comorbidity index among covariates.
Results were reported as adjusted coefficient (Coeff), adjusted hazard ratio (HR), 95% confidence intervals (95%CI), and p values, as appropriate. Statistical analyses were performed using Stata 15.0. Results were considered statistically significant for p < 0.05.
Results
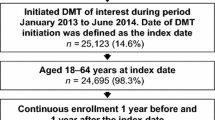
From the population of people with MS in the Campania Region from 2015 to 2020 (n = 7080), we included 2495 individuals who were commenced on a DMT from 2018 to 2020, corresponding to 2918 ITPs (the same individual being treated with different DMTs within the study period). Reasons for exclusion are reported in Fig. 1. Demographics, comorbidities and treatment features of included patients (and respective ITPs) are reported in Table 1.
Overall, we included 398 patients treated with ocrelizumab, corresponding to 399 ITPs. Looking at administration route, we included 293 ITPs with other infusion DMTs (alemtuzumab, natalizumab), 1325 with oral DMTs (cladribine, fingolimod, teriflunomide, dimethyl fumarate), and 901 with injectable DMTs (glatiramer acetate, interferon beta-1a, interferon beta-1b, and peg-interferon beta-1a). Looking at efficacy line, we included 724 ITPs with other highly active DMTs (alemtuzumab, natalizumab, cladribine, fingolimod), and 1795 with low/medium efficacy DMTs (teriflunomide, dimethyl fumarate, interferon beta-1a, interferon beta-1b, and peg-interferon beta-1a). Most frequently prescribed DMTs were dimethyl fumarate (n = 588, 20.1%), fingolimod (n = 401, 13.7%) and ocrelizumab (n = 399, 13.6%), with ocrelizumab being the most frequently prescribed DMT in 2019. Also, we observed an overall drop in new DMT prescriptions in 2020 (Table 1).
Most patients treated with ocrelizumab were newly diagnosed and drug naïve (n = 104), followed by patients previously treated with fingolimod (n = 76), dimethyl fumarate (n = 54), teriflunomide (n = 51), glatiramer-acetate (n = 37), natalizumab (n = 34), interferon beta1a (n = 16), interferon beta1b (n = 13), alemtuzumab (n = 12), and peg-interferon beta1a (n = 2).
ITP durations and number of patients switching to other DMT or completely discontinuing DMTs are reported in Table 2. A minority of ocrelizumab ITPs was discontinued (4 over 399), after 13.71 ± 5.42 months; in particular, 1 patient was switched to natalizumab, 2 patients to dimethyl fumarate, and 1 patient to interferon beta1a. When compared with ocrelizumab, the risk of discontinuation was higher for other infusion (HR = 3.64; 95%CI = 1.18, 11.18; p = 0.02), oral (HR = 9.19; 95%CI = 3.29, 25.65; p < 0.01) and injectable DMTs (HR = 5.54; 95%CI = 1.96, 15.08; p < 0.01) (Fig. 2a). Similarly, when compared with ocrelizumab, the risk of discontinuation was higher for other highly active (HR = 3.78; 95%CI = 1.33, 10.76; p = 0.01), and low/medium efficacy DMTs (HR = 7.59; 95%CI = 2.75, 20.95; p < 0.01) (Fig. 2b). Results were confirmed also after adjusting by Charlson Comorbidity index.
Kaplan–Meier estimates of treatment persistence. Adjusted hazard ratio (HR), coefficients (Coeff) and p-values are shown from Cox regression models evaluating administration route (a) and clinical efficacy (b), and including age, sex, year of treatment start (2018, 2019, or 2020), treatment duration, and adherence as covariates
Adherence to treatment is reported in Table 3. When compared with ocrelizumab, adherence (PDC) was lower for oral DMTs (Coeff = − 0.18; 95%CI = − 0.26, − 0.12; p < 0.01), but similar to other infusion (Coeff = − 0.08; 95%CI = − 0.19, 0.02; p = 0.14), and injectable DMTs (Coeff = − 0.01; 95%CI = − 1.11, 0.11; p = 0.90). When compared with ocrelizumab, adherence was lower for other highly active DMTs (Coeff = − 0.11; 95%CI = − 0.19, − 0.02; p < 0.01), and low/medium efficacy DMTs (Coeff = − 0.18; 95%CI = − 0.26, − 0.10; p < 0.01). Results were confirmed also after adjusting by Charlson Comorbidity index.
Healthcare resource utilization and costs are reported in Table 4. When compared with ocrelizumab, AHR was higher for other infusion DMTs (Coeff = 0.05; 95%CI = 0.01, 0.09; p = 0.03), and similar to oral (Coeff = − 0.01; 95%CI = − 0.03, 0.03; p = 0.97) and injectable DMTs (Coeff = 0.01; 95%CI = − 0.02, 0.05; p = 0.45). When compared with ocrelizumab, AHR was similar to other highly active (Coeff = 0.01; 95%CI = − 0.02, 0.04; p = 0.51), and low/medium efficacy DMTs (Coeff = 0.01; 95%CI = − 0.02, 0.04; p = 0.55). Results were confirmed also after adjusting by Charlson Comorbidity index.
When compared with ocrelizumab, monthly costs for MS hospital admissions were similar to other infusion DMTs (Coeff = 7.83; 95%CI = − 12.94, 28.61; p = 0.46), but lower for oral (Coeff = − 18.95; 95%CI = − 35.27, − 2.64; p < 0.01) and injectable DMTs (Coeff = − 28.25; 95%CI = − 46.44, − 2.64; p = 0.02). When compared with ocrelizumab, monthly costs for MS hospital admissions were similar to other highly active (Coeff = − 0.77; 95%CI = − 18.12, 16.57; p = 0.93), but lower for low/medium efficacy DMTs (Coeff = − 26.02; 95%CI = − 42.45, − 9.58; p < 0.01). Results were confirmed also after adjusting by Charlson Comorbidity index.
When compared with ocrelizumab, monthly costs were similar to other infusion DMTs (Coeff = − 57.28; 95%CI = − 119.15, 4.59; p = 0.07), but lower for oral (Coeff = − 675.83; 95%CI = − 723.16, − 628.50; p < 0.01) and injectable DMTs (Coeff = − 675.83; 95%CI = − 1205.92, − 1100.75; p < 0.01). When compared with ocrelizumab, monthly costs were higher for other highly active DMTs (Coeff = 92.30; 95%CI = 53.01, 131.60; p < 0.01), but lower for low/medium efficacy DMTs (Coeff = − 1043.61; 95%CI = − 1080.02, − 1007.20; p < 0.01). Results were confirmed also after adjusting by Charlson Comorbidity index.
Discussion
In this population-based study, we specifically aimed to describe the use of ocrelizumab in the real-world of the Campania Region of Italy, with regards to prescription pattern, persistence, adherence, healthcare resource utilization and related costs. Ocrelizumab was the most frequently prescribed DMT for MS in 2019, with 26% prescriptions being made to treatment-naïve MS patients, suggesting it was addressing unmet needs in the MS treatment scenario. This is the first real-world study on ocrelizumab describing both utilization pattern (i.e., persistence, adherence), and related healthcare resource utilization and costs.
When compared with other high-efficacy DMTs, ocrelizumab was used on much more complex populations (i.e., older age, higher comorbidity burden), as already described by some previous studies [17,18,19]. Notwithstanding this, in our cohort, only 1% patients were discontinued from ocrelizumab, suggesting optimal efficacy and safety [20, 21], with higher persistence rates compared with other oral, infusion and injectable DMTs. This could be at least in part due to the use of ocrelizumab on newly diagnosed and treatment-naïve patients in our cohort, which is a known factor of optimal treatment response [5]. Ocrelizumab has already proved high persistence rates in previous studies [12, 19, 22, 23], with efficacy and safety issues being the most common causes of discontinuation [19, 23]. Of note, relapses, disability progression and MRI activity are expected to occur in a minority of patients treated with ocrelizumab [17, 18, 24]. Taken together, our data suggest that ocrelizumab high persistence rates might be a consequence of optimal efficacy and safety.
We also found high rates of adherence to ocrelizumab compared with lower and similar efficacy class. While we have to acknowledge that adherence analyses were run on the subset of patients with at least 12 months of follow-up, our rate of adherence is in line with previous similar studies [12, 25], and overall suggests optimal safety profile (e.g., no need to delay infusions). Looking at previous real-world studies, side effects were reported by 10% of patients, mostly consisting of mild infusion-related reactions and infections [18], independently from age [17].
The main novelty of our study is the inclusion of healthcare resource utilization and costs. In particular, ocrelizumab was associated with lower direct treatment costs, but was associated with similar probability of MS-related hospital admissions and costs, when compared with other DMTs similar in administration route (e.g., natalizumab, alemtuzumab) and efficacy class (e.g., natalizumab, alemtuzumab, cladribine, fingolimod). Similarly, in a previous US claims’ study including 189 patients treated with ocrelizumab, alemtuzumab or natalizumab for 1 year, authors showed reduced costs for ocrelizumab treatment and related procedures [26]. Overall, these findings suggest that ocrelizumab is less expensive but similarly effective to other high-efficacy DMTs.
Limitations of our study include the generalizability of our results, since we only included patients from a specific Italian region. However, our cohort had similar distribution (e.g., age, DMT use), when compared to other international studies [17, 18, 27], and, hence, may reflect the general MS population treated with ocrelizumab. Also, rates of disability progression, relapses and related healthcare resource utilization are expected to increase over the follow-up [28]. Therefore, longer follow-up is warranted to confirm our findings. In addition, we have also included 2020 year in the analysis, with the bias of COVID19 pandemic that could have caused extended interval dosing for ocrelizumab [29]; however, based on adherence results, this was not the case for most infusions and the drop of new prescriptions in 2020 is in line with a previous English study [30]. Our study also holds limitations derived from the use of routinely collected healthcare data, including the definition of MS-related hospital admission based on the primary diagnosis that could be biased by the physician perspective. We compared ocrelizumab to other approved DMTs specifically approved for MS, while did not extend the analysis to other treatments (e.g., rituximab) due to sample size constraints and possible selection bias, deriving from their use in highly selected populations (e.g., non-responders to approved DMTs).
In conclusion, we confirmed previous results on high persistence and adherence rates of ocrelizumab, when compared with DMTs of similar efficacy and mode of administration. We also showed that ocrelizumab is less expensive than other high-efficacy DMTs, while possibly equally effective based on indirect measures on routinely collected healthcare data (i.e., AHR and related costs).
References
Montalban X, Hauser SL, Kappos L et al (2017) Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 376:209–220. https://doi.org/10.1056/NEJMoa1606468
Hauser SL, Bar-Or A, Comi G et al (2017) Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 376:221–234. https://doi.org/10.1056/NEJMoa1601277
Wolinsky JS, Arnold DL, Brochet B et al (2020) Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol 19:998–1009. https://doi.org/10.1016/S1474-4422(20)30342-2
Hauser SL, Kappos L, Montalban X et al (2021) Safety of ocrelizumab in patients with relapsing and primary progressive multiple sclerosis. Neurology 97:E1546–E1559. https://doi.org/10.1212/WNL.0000000000012700
Lanzillo R, Carotenuto A, Signoriello E et al (2022) Prognostic markers of ocrelizumab effectiveness in multiple sclerosis : a real world observational multicenter study. J Clin Med 11:2081. https://doi.org/10.3390/jcm11082081
Capasso N, Nozzolillo A, Scalia G et al (2021) Ocrelizumab depletes T-lymphocytes more than rituximab in multiple sclerosis. Mult Scler Relat Disord 49:102802. https://doi.org/10.1016/j.msard.2021.102802
Glaser A, Stahmann A, Meissner T et al (2019) Multiple sclerosis registries in Europe—an updated mapping survey. Mult Scler Relat Disord 27:171–178. https://doi.org/10.1016/j.msard.2018.09.032
Moccia M, Affinito G, Ronga B et al (2022) Emergency medical care for multiple sclerosis: a five-year population study in the Campania Region (South Italy). Mult Scler 28:597–607. https://doi.org/10.1177/13524585221074010
De Angelis F, John N, Brownlee W (2018) Disease-modifying therapies for multiple sclerosis. BMJ 363:1–10. https://doi.org/10.1136/bmj.k4674
Birnbaum HG, Ivanova JI, Samuels S et al (2009) Economic impact of multiple sclerosis disease-modifying drugs in an employed population: direct and indirect costs. Curr Med Res Opin 25:869–877. https://doi.org/10.1185/03007990902743869
Moccia M, Brescia Morra V, Lanzillo R et al (2020) Multiple sclerosis in the campania region (South Italy): algorithm validation and 2015–2017 prevalence. Int J Env Res Public Heal 17:3388. https://doi.org/10.3390/ijerph17103388
Engmann N, Sheinson D, Bawa K et al (2021) Persistence and adherence to ocrelizumab compared with other disease-modifying therapies for multiple sclerosis in U.S. commercial claims data. J Manag Care Spec Pharm. https://doi.org/10.18553/jmcp.2021.20413
Moccia M, Loperto I, Lanzillo R et al (2020) Persistence, adherence, healthcare resource utilisation and costs for interferon Beta in multiple sclerosis: a population-based study in the Campania region (southern Italy). BMC Health Serv Res 20:797. https://doi.org/10.1186/s12913-020-05664-x
Agenzia Italiana del Farmaco (2021) Banca Dati Farmaci dell’AIFA. https://www.farmaci.agenziafarmaco.gov.it/
Moccia M, Affinito G, Capacchione A et al (2021) Interferon beta for the treatment of multiple sclerosis in the Campania Region of Italy: merging the real-life to routinely collected healthcare data. PLoS ONE 16:e0258017. https://doi.org/10.1371/journal.pone.0258017
Charlson M, Pompei P, Ales K, MacKenzie C (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. https://doi.org/10.1016/0021-9681(87)90171-8
Smoot K, Chen C, Stuchiner T et al (2021) Clinical outcomes of patients with multiple sclerosis treated with ocrelizumab in a US community MS center: an observational study. BMJ Neurol Open 3:1–7. https://doi.org/10.1136/bmjno-2020-000108
Pontieri L, Blinkenberg M, Bramow S et al (2022) Ocrelizumab treatment in multiple sclerosis: a Danish population-based cohort study. Eur J Neurol 29:496–504. https://doi.org/10.1111/ene.15142
Meuth S, Buttmann M, Weber M et al (2020) Real-world persistence and adherence to ocrelizumab in patients with multiple sclerosis over 24 months—interim analysis of the CONFIDENCE study. ECTRIMS
Moccia M, Palladino R, Carotenuto A et al (2016) Predictors of long-term interferon discontinuation in newly diagnosed relapsing multiple sclerosis. Mult Scler Relat Disord 10:90–96. https://doi.org/10.1016/j.msard.2016.09.011
Lanzillo R, Prosperini L, Gasperini C et al (2018) A multicentRE observational analysiS of PErsistenCe to treatment in the new multiple sclerosis era: the RESPECT study. J Neurol. https://doi.org/10.1007/s00415-018-8831-x
Pardo G, Pineda ED, Ng CD et al (2022) Adherence to and persistence with disease-modifying therapies for multiple sclerosis over 24 months: a retrospective claims analysis. Neurol Ther 11:337–351. https://doi.org/10.1007/s40120-021-00319-3
Rojas JI, Patrucco L, Fruns M et al (2021) Real-world experience of ocrelizumab in multiple sclerosis patients in latin america. Arq Neuropsiquiatr 79:305–309. https://doi.org/10.1590/0004-282X-ANP-2020-0339
Fernandez-Diaz E, Perez-Vicente JA, Villaverde-Gonzalez R et al (2021) Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann Clin Transl Neurol 8:385–394. https://doi.org/10.1002/acn3.51282
Pineda E, Ng C, Sheinson D et al (2021) Adherence and persistence to disease-modifying therapies for multiple sclerosis and their impact on clinical and economic outcomes in a US claims database. AAN
Nicholas J, Halpern R, Ziehn M et al (2020) Real-world cost of treatment for multiple sclerosis patients initiating and receiving infused disease-modifying therapies per recommended label in the United States. J Med Econ 23:885–893. https://doi.org/10.1080/13696998.2020.1761821
Bossart J, Kamm CP, Kaufmann M et al (2022) Real-world disease-modifying therapy usage in persons with relapsing-remitting multiple sclerosis: cross-sectional data from the Swiss Multiple Sclerosis Registry. Mult Scler Relat Disord 60:103706. https://doi.org/10.1016/j.msard.2022.103706
Cellerino M, Boffa G, Lapucci C et al (2021) Predictors of ocrelizumab effectiveness in patients with multiple sclerosis. Neurotherapeutics 18:2579–2588. https://doi.org/10.1007/s13311-021-01104-8
Rolfes L, Pawlitzki M, Pfeuffer S et al (2021) Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol Neuroimmunol neuroinflammation 8:1–9. https://doi.org/10.1212/NXI.0000000000001035
Williams T, Mishra R, Bharkhada B et al (2022) Impact of the COVID-19 pandemic on the prescription of disease-modifying therapy for multiple sclerosis in England: a nationwide study. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2021-328340
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. Roche (Grant no. 2021)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by Roche SpA, Monza, Italy, on the basis of a Sponsored Research Agreement. Marcello Moccia has received research grants from ECTRIMS-MAGNIMS, UK MS Society, and Merck; honoraria from Biogen, BMS Celgene, Merck, Roche, and Sanofi-Genzyme. Antonio Carotenuto has received research grants from Almirall, research grants from ECTRIMS-MAGNIMS and honoraria from Almirall, Biogen, Roche Sanofi-Genzyme and Novartis. Maria Petracca has received research grants from Italian MS Foundation and Baroni Foundation, honoraria from HEALTH&LIFE S.r.l. and Biogen and sponsorship for travel/meeting expenses from Novartis, Roche and Merck. Roberta Lanzillo has received honoraria from Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. Vincenzo Brescia Morra has received research grants from the Italian MS Society, and Roche; and honoraria from Bayer, Biogen, Merck, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva. Other authors have nothing to disclose.
Ethical standard statement
The study was approved by the Federico II Ethics Committee (355/19).The study was performed in accordance with good clinical practice and Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moccia, M., Affinito, G., Berera, G. et al. Persistence, adherence, healthcare resource utilization and costs for ocrelizumab in the real-world of the Campania Region of Italy. J Neurol 269, 6504–6511 (2022). https://doi.org/10.1007/s00415-022-11320-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11320-7