Abstract
Introduction
Diffusion tensor imaging (DTI) can assess the structural integrity of the corticospinal tract (CST) in vivo. We aimed to investigate whether CST DTI metrics after intracerebral haemorrhage (ICH) are associated with 6-month functional outcome and can improve the predictive performance of the existing ICH score.
Methods
We retrospectively included 42 patients with DTI performed within 5 days after deep supratentorial spontaneous ICH. Ipsilesional-to-contralesional ratios were calculated for fractional anisotropy (rFA) and mean diffusivity (rMD) in the pontine segment (PS) of the CST. We determined the most predictive variables for poor 6-month functional outcome [modified Rankin Scale (mRS) > 2] using the least absolute shrinkage and selection operator (LASSO) method. We calculated discrimination using optimism-adjusted estimation of the area under the curve (AUC).
Results
Patients with 6-month mRS > 2 had lower rFA (0.945 [± 0.139] vs 1.045 [± 0.130]; OR 0.004 [95% CI 0.00–0.77]; p = 0.04) and higher rMD (1.233 [± 0.418] vs 0.963 [± 0.211]; OR 22.5 [95% CI 1.46–519.68]; p = 0.02). Discrimination (AUC) values were: 0.76 (95% CI 0.61–0.91) for the ICH score, 0.71 (95% CI 0.54–0.89) for rFA, and 0.72 (95% CI 0.61–0.91) for rMD. Combined models with DTI and non-DTI variables offer an improvement in discrimination: for the best model, the AUC was 0.82 ([95% CI 0.68–0.95]; p = 0.15).
Conclusion
In our exploratory study, PS-CST rFA and rMD had comparable predictive ability to the ICH score for 6-month functional outcome. Adding DTI metrics to clinical-radiological scores might improve discrimination, but this needs to be investigated in larger studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous (non-traumatic) intracerebral haemorrhage (ICH) is a severe form of stroke, accounting for nearly 3 million deaths globally in 2017 [1]. About 40% of patients die within the first month, while about 80% of those who survive are functionally dependent on others [2]. Given the frequently poor clinical outcome after ICH, clinicians are often faced with difficult decisions regarding the appropriate level of acute treatment, for example admission to critical care or neurosurgery. Early functional outcome prediction after ICH is thus fundamental in everyday clinical practice to guide subsequent care. Many clinical-radiological prognostic scores have been proposed, of which the ICH score is one of the most validated, being initially designed to predict 30-day mortality [3]. The ICH score ranges from 0 to 6, including simple clinical and radiological variables: GCS score (2 point if GCS 3 to 4 and 1 point if GCS score 5–12), age (1 point if ≥ 80 years), ICH site (1 point for infratentorial origin), ICH volume (1 point if ≥ 30 cm3) and the presence of intraventricular haemorrhage (1 point if present). The ICH score has modest discrimination performance for functional outcome (typical c-index around 0.70–0.80), as do other similar prognostic instruments [4]. Most available studies predict mortality at 30 days or 3-months functional outcome, with decreasing predictive performance as the time interval from the index event increases [4]. However, functional improvement after ICH can occur beyond 3-months [5, 6], and full recovery from intracerebral haemorrhage may take longer than that from acute ischaemic stroke; a reliable 6-month functional outcome prediction instrument would therefore be very useful for clinicians in the acute phase of ICH.
The modified Rankin Scale (mRS) [7]—a 6-point scale with possible disability scores ranging from 0 (no residual symptoms) to 5 (severe disability, bedridden, requires continuous care), and with score 6 for patients who have died—is strongly related to motor recovery which, in turn, is likely to be dependent on the integrity of the corticospinal tract (CST) [8, 9].
Diffusion tensor imaging (DTI), as a measure of brain microstructure, is thus a promising biomarker to predict long-term motor outcome after both ischaemic stroke [10] and ICH [11]. Based on fractional anisotropy (FA) and mean diffusivity (MD) changes, DTI provides quantitative in vivo information regarding the structural integrity of brain tissue including white matter tracts, even where these appear normal on standard structural MRI. Several studies have examined whether DTI-derived data, applied to assess the integrity of CST, can predict motor outcome after ICH. However, these studies were methodologically heterogeneous and focused on early (3 months) outcome and have provided inconsistent results. Furthermore, most studies did not investigate the added predictive value of DTI in addition to conventional clinical scores [11].
We therefore assessed the integrity of the pontine segment of CST (PS-CST) via DTI in the early phase (within 5 days) after symptom onset in patients with deep supratentorial ICH. We included only this ICH location because of its proximity and likely consistent impact on the fibres of the ipsilateral CST; by contrast, lobar ICH may have an erratic impact on CST, while infratentorial ICH may be located within the PS-CST. We aimed to investigate the predictive performance of early-phase PS-CST DTI metrics acquired in the first 5 days from ICH onset, both alone and when added to the ICH score.
Methods
Study population
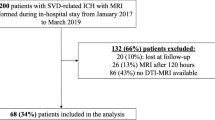
We retrospectively included consecutive patients with first-ever deep supratentorial ICH (with no previous history of ICH or ischaemic stroke) from the prospective SIGNaL (Stroke InvestiGation in North and Central London) registry who presented from January 2017 to March 2019. Other inclusion criteria were: 6-month follow-up data available and DTI sequences (of adequate quality for analysis) acquired within 5 days after ICH onset. We excluded patients with secondary causes of ICH (tumour, vascular malformation, aneurysm, vasculitis, venous infarction or haemorrhagic transformation of an infarct), history of trauma, or ICH located in lobar or infratentorial regions.
Clinical evaluation
We retrieved baseline detailed demographic, clinical and radiological information. Six-month functional outcome was assessed via mRS at follow-up visits or by phone call. Data were collected as part of routine clinical care, and data analysis was approved as a service evaluation by the University College London Hospital Trust Data Governance Review Board.
MRI imaging acquisition
All MRI scans were performed on a Philips Achieva 3 Tesla scanner (Philips, Best, Netherlands). The following acquisitions were included: single-shell diffusion-weighted imaging (DWI) (voxel resolution 0.9 × 0.9 × 5 mm3, echo time 76 ms, repetition time 3.5 s, flip angle 90°) comprising one b = 0, followed by six b = 1000 s/mm2 volumes; T1-weighted imaging (voxel resolution 0.94 × 0.94 × 1.1 mm3, echo time 3.3 ms, repetition time 7.1 ms, flip angle 9°); fluid-attenuated inversion recovery (FLAIR) imaging (voxel resolution 0.45 × 0.45 × 4 mm3, echo time 110 ms, repetition time 10.8 s, inversion time 2.8 s, flip angle 90°); and susceptibility-weighted imaging (SWI) (voxel resolution 0.24 × 0.24 × 1 mm3, repetition time 31 ms, flip angle 17°).
MRI analysis and lesion segmentation
Every MRI was evaluated by a single rater (GS) blinded to other clinical variables. ICH and peri-haematomal oedema (PHE) regions were manually segmented on SWI and FLAIR sequences, respectively. The regions of interest (ROI) obtained from ICH and PHE segmentations were used to obtain the ICH and PHE volumes. ICH location was assessed using the Cerebral Haemorrhage Anatomical Rating Instrument (CHARTS) [12]. The ICH score was calculated for every patient according to the original publication [3].
MRI image processing and DTI metrics
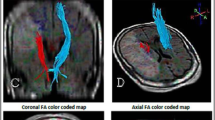
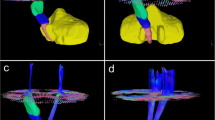
DWI data were corrected for eddy currents prior to DTI fitting using FSL [13]. ROIs for the CST and the PS-CST were obtained using the John Hopkins University [JHU] DTI-based white matter atlas (https://identifiers.org/neurovault.image:1401) (Fig. 1) non-rigidly transformed to each subject’s DWI image space. Similarly, to obtain the ICH and PHE ROIs in each subject’s DWI space, non-rigid transformations were computed between SWI and DWI, and between FLAIR and DWI image spaces. All image transformations were done using the NiftyReg software package [14]. Finally, mean FA and MD were computed in the CST and in the PS-CST using the obtained ROIs. To obtain the ICH probability map, we summed all the lesion masks and divided by the number of patients to give a lesion probability at each voxel.
Statistical analysis
Ipsilesional-to-contralesional PS-CST ratios were calculated for FA (rFA = FA affected side/FA unaffected side) and MD (rMD = MD affected side / MD in unaffected side). Long-term (6 months) functional outcome was defined as poor if mRS was greater than 2. The association between clinical and radiological variables and poor 6-month functional outcome was assessed via univariable logistic regression analysis. rFA, rMD and ICH score discrimination for poor functional outcome was assessed by calculating the area under the ROC (receiver operating characteristic) curve (AUC). After selection of DTI (rFA and rMD) and non-DTI predictors (standard clinical and radiological variables) from univariable analyses (p < 0.10), we fitted logistic regression models using least absolute shrinkage and selection operator (LASSO) estimation [15] to find variables with best predictive ability for poor mRS at 6 months. Two different models were obtained: Model 1 combined DTI plus non-DTI variables, while Model 2 combined DTI plus the ICH score. We validated the models using bootstrapping and calculated optimism-adjusted estimates of the AUC [16]. We fitted both models (using standard logistic regression)-both including the ICH score, to obtain nested models - and compared the fits using the likelihood ratio (LR) test. Statistical analysis was performed using STATA 16 (StataCorp, College Station, TX). The statistical significance level was set at p = 0.05.
Results
We included 42 adult patients with spontaneous supratentorial deep ICH (flow chart in Fig. 2). Group probability maps showed the ICH lesion distribution reported in Fig. 3. Table 1 summarizes clinical and radiological characteristics in the entire cohort, including PS-CST mean rFA and rMD. Median age was 62 years (IQR 52–72), and median ICH volume was 5.4 mL (IQR 3.0–11.7). Thirteen patients (31.0%) had poor functional outcome (mRS > 2) at 6 months. Mean rFA was 1.024 (SD 0.139), and mean rMD was 1.046 (SD 0.312). Table 2 describes univariable logistic regression models for the association between clinical and radiological variables, including rFA and rMD, and poor long-term functional outcome.
Patients with poor functional outcome were older (median age 72 [IQR 62–80] vs 60 [IQR 49–68]; OR per 1 year increase 1.06 [1.00–1.12]; p = 0.039) and had higher ICH scores (OR 13.87 for score 1 vs score 0 [95% CI 2.70–71.20]; p = 0.002). Intraventricular haemorrhage (IVH) was associated with poor functional outcome (OR 24.0 [95% CI 2.47–233.06]; p = 0.006). Patients with poor long-term mRS had significantly lower rFA (0.945 [± 0.139] vs 1.045 [± 0.130]; OR 0.004 [95% CI 0.00–0.77]; p = 0.04) and significantly higher rMD (1.233 [± 0.418] vs 0.963 [± 0.211]; OR 22.5 [95% CI 1.46–519.68]; p = 0.02) in the PS-CST.
The discrimination of the ICH score for poor functional outcome at 6-months, measured by the AUC, was 0.76 (95% CI 0.61–0.91); discrimination for rFA and rMD was 0.71 (95% CI 0.54–0.89) and 0.72 (95% CI 0.61–0.91), respectively.
We built two different LASSO regression models (Table 3). In Model 1, we included both DTI (rFA and rMD) and non-DTI metrics (age and IVH): all the variables included in the model were selected via LASSO regression analysis, and the optimism-adjusted predictive ability for poor mRS at 6 months was 0.81 (95% CI 0.69–0.94). In Model 2, we included both DTI variables (rFA and rMD) and ICH score; rMD and ICH score were selected, and the optimism-adjusted AUC for this model was 0.82 (95% CI 0.68–0.95). The LR test to evaluate the statistical significance of the difference in the predictive ability between Model 1 and Model 2 against the ICH score alone gave p values of 0.13 and 0.15, respectively.
Discussion
Our study confirmed the feasibility of using DTI in the acute phase of ICH to extract metrics that can quantify altered microstructure in CST, which is associated with 6-month functional outcome. Predictive performances of DTI metrics alone were comparable to the existing ICH prognostic score (PS-CST rFA 0.71 [95% CI 0.54–0.89] and PS-CST rMD 0.72 [95% CI 0.53–0.92] versus ICH score 0.76 [95% CI 0.61–0.91]). This exploratory study suggests that a model with DTI and non-DTI variables might offer an improvement in the prediction of 6-month functional outcome. However, a larger study is required to investigate this further and determine whether any improvement is statistically significant.
Our findings are consistent with a limited number of previous studies [17,18,19,20] , in which DTI metrics were also associated with outcome after ICH. However, given the great methodological heterogeneity among the available studies—related to differences in the study cohorts, the time-period between index event and brain MRI, data acquisition and processing methods, anatomical site of DTI measurements, and outcome measures—the potential clinical benefit of the application of DTI in ICH has been difficult to assess.
Only one study has compared the predictive performance of DTI parameters and the ICH score [21] and found that the prognostic value of the ICH score substantially surpassed that of CST-related DTI metrics (AUC 0.74 vs. 0.44; p = 0.01 for mRS > 2); moreover, the combination of the DTI metrics with the ICH score did not improve the prognostication of outcome. Our findings appear more promising regarding the potential predictive clinical value of DTI metrics; our estimates suggest that combined models with DTI and non-DTI variables (including ICH score) might provide better outcome discrimination than ICH score alone, although the difference in discrimination was not statistically significant. The discrepancy might be due to differences in population characteristics: 68.8% of patients included in the cited study had lobar ICH, and given their variable location and impact on CST fibres, lobar ICH is likely to have less consistent impact on CST. By contrast, we included only patients with deep supratentorial ICH; as shown in Fig. 3, the ICH location in our cohort included putamen, globus pallidus, internal capsule, thalamus and external capsule. Haemorrhage and peri-haematomal oedema in these regions are expected to consistently affect the CST via both direct disruption or displacement of fibre tracts and indirect mechanisms including oedema, inflammation and early Wallerian degeneration. Our study suggests that in acute ICH, DTI metrics in the pons can detect microstructural alterations of the CST which are associated with 6-month mRS.
The timing of measuring microstructural changes in DTI is of potential importance because Wallerian degeneration of CST following acute insult is a dynamic evolving phenomenon. [22] A recent study [23] on mixed (lobar and deep) ICH found no changes in FA at the cerebral peduncle within 12 h after ICH, which might have been due to the ultra-early timing of MRI acquisition in the study. By contrast, we included patients with MRI performed within 5 days after ICH; in the vast majority of patients (95.2%), MRI was performed at least 24 h after the index event. These findings suggest that DTI metrics might be of greater predictive value if measured beyond the hyperacute phase of ICH. On the other hand, our study demonstrated that DTI parameters are predictive of functional outcome even at an early stage, in which often some important clinical decisions must be made in ICH patients (e.g. in relation to admission to neurocritical care or consideration of neurosurgery).
Our findings suggest that quantitative MRI (DTI) is feasible and of potential clinical predictive value that could add information beyond that from standard neuroimaging in acute ICH. Direct anatomical involvement of the CST due to haematoma mass effect or destruction is associated with functional outcome after ICH [24], which can be detected by standard structural neuroimaging techniques. However, peri-haematomal oedema, inflammation and early Wallerian degeneration could cause functionally relevant injury to white matter fibres beyond the haematoma itself; our data indicate that DTI provides a quantitative measure of CST integrity related to these mechanisms with potential to add valuable prognostic information to that available from standard structural MRI.
The brain MRIs in our study were performed as part of standard clinical acquisition protocols and did not lengthen or reduce tolerability of the examination. Brain MRI already plays a key role in ICH-related diagnostic work-up in practice; it is widely performed to seek evidence of underlying structural causes (e.g. tumours, cavernomas, macrovascular lesions) and is more sensitive than CT for visualizing biomarkers of the cerebral small vessel diseases that cause most ICH (i.e. white matter hyperintensities, cerebral microbleeds, lacunes and perivascular spaces). The potential predictive ability of DTI metrics, in combination with standard clinical and radiological variables, may further consolidate the role of MRI in acute ICH.
The modified Rankin Scale is the most widely functional outcome scale adopted in stroke studies but is highly oriented on motor outcome which is highly dependent on CST integrity. However, the clinical impact of ICH goes far beyond motor outcome, including cognitive deficits and dementia [25, 26]. Similarly, the role of DTI might go beyond the measurement of Wallerian degeneration in CST. Several studies have already adopted a whole brain approach and have evaluated the association between DTI parameters and cognitive functions [27]. The application of a whole-brain DTI approach to predict ICH functional outcomes is thus potentially of interest for future research.
Our study has strengths. Our population is homogeneous with respect to the timing of MRI, site of ICH and standardized DTI region of interest (PS-CST). A single-rater assessment of ICH and PHE lesions reduced variability in the segmentation process. We also applied a standardized automated post-processing protocol. In view of the modest sample size, we used LASSO estimation, a technique that produces better models for prediction in small datasets.
We also acknowledge limitations. The small cohort size limits the precision of our risk estimates with wide 95% CI, but to the best of our knowledge this is the largest study of deep supratentorial ICH in a Western population. We do not have detailed information on the pattern or severity of motor deficit on hospital admission; however, since all participants had deep ICH and were admitted with clinical symptoms suggesting stroke, it is highly likely that a motor deficit was present in the great majority of those included. A potential advantage of our study is that the findings are generalizable to patients regardless of the severity of their initial motor deficit, which can in any case fluctuate markedly in the first few days after ICH. Although MRI studies are routinely performed for patients with ICH and no contraindications in our centre, the requirement for MRI is still likely to have created a selection bias: the study population includes clinically milder ICH with small hematoma volumes (only 2 patients with baseline GCS < 15 and median ICH volume 5.4 ml [IQR 3.0–11.7]). These aspects might limit the generalizability of our findings to patients with more severe ICH. Nevertheless, the AUC we found for the ICH score is similar to those from studies that evaluated 6-month functional outcome in heterogeneous ICH populations (pooled AUC from four studies: 0.78 [95% CI 0.74–0.82])[4], suggesting that our prediction models may be generalizable to other ICH populations. The mRS, although in part related to motor function, is also affected by other neurological impairments not related to the CST (e.g. cognition); thus, evaluation of whole-brain DTI measures in future studies might improve associations with functional outcome.
Conclusion
In the acute phase after deep supratentorial spontaneous ICH, DTI is feasible as part of routine clinical care; rFA and rMD measured in the normal-appearing pontine segment of the corticospinal tract demonstrated acceptable prognostic ability for 6-month functional outcome. Our findings suggest that DTI measures show promise to improve early prognostication after acute ICH, but further studies in larger cohorts are needed.
Data sharing and availability statement
All de-identified participant data requests should be submitted to the corresponding author for consideration by the SIGNaL Steering Committee.
References
GBD 2017 Causes of Death Collaborators (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1736–1788. https://doi.org/10.1016/S0140-6736(18)32203-7
van Asch CJ, Luitse MJA, Rinkel GJE et al (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9(2):167–176. https://doi.org/10.1016/S1474-4422(09)70340-0
Hemphill JC, Bonovich DC, Besmertis L et al (2001) The ICH Score. Stroke 32(4):891–897. https://doi.org/10.1161/01.str.32.4.891
Gregório T, Pipa S, Cavaleiro P et al (2019) Assessment and comparison of the four most extensively validated prognostic scales for intracerebral hemorrhage: systematic review with meta-analysis. Neurocrit Care 30(2):449–466. https://doi.org/10.1007/s12028-018-0633-6
Lee KB, Kim JS, Hong BY, Kim YD et al (2015) The motor recovery related with brain lesion in patients with intracranial hemorrhage. Behav Neurol. https://doi.org/10.1155/2015/258161
Sreekrishnan A, Leasure AC, Shi FD et al (2017) Functional improvement among intracerebral hemorrhage (ICH) survivors up to 12 months post-injury. Neurocrit Care 27(3):326–333. https://doi.org/10.1007/s12028-017-0425-4
van Swieten JC, Koudstaal PJ, Visser MC et al (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19(5):604–607. https://doi.org/10.1161/01.str.19.5.604
Hendricks HT, van Limbeek J, Geurts AC et al (2002) Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 83(11):1629–1637. https://doi.org/10.1053/apmr.2002.35473
Lindenberg R, Renga V, Zhu LL et al (2010) Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 74(4):280–287. https://doi.org/10.1212/WNL.0b013e3181ccc6d9
Werring DJ, Toosy AT, Clark CA et al (2000) Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 69(2):269–272. https://doi.org/10.1136/jnnp.69.2.269
Moura LM, Luccas R, de Paiva JPQ et al (2019) Diffusion tensor imaging biomarkers to predict motor outcomes in stroke: a narrative review. Front Neurol 8(10):445. https://doi.org/10.3389/fneur.2019.00445
Charidimou A, Schmitt A, Wilson D et al (2017) The Cerebral Haemorrhage Anatomical RaTing inStrument (CHARTS): Development and assessment of reliability. J Neurol Sci 15(372):178–183. https://doi.org/10.1016/j.jns.2016.11.021
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62(2):782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Modat M (2012) Inverse-consistent symmetric free form deformation. In: Dawant BITM, Christensen GE, Fitzpatrick JM, Rueckert D (eds) Biomedical image registration. Springer Berlin Heidelberg, pp 79–88
Ambler G, Brady AR, Royston P (2002) Simplifying a prognostic model: a simulation study based on clinical data. Stat Med 21(24):3803–3822. https://doi.org/10.1002/sim.1422
Harrel FE (2015) Regression modeling strategies. Springer
Yoshioka H, Horikoshi T, Aoki S et al (2008) Diffusion tensor tractography predicts motor functional outcome in patients with spontaneous intracerebral hemorrhage. Neurosurgery 62(1):97–103. https://doi.org/10.1227/01.NEU.0000311066.03121.B8
Kusano Y, Seguchi T, Horiuchi T et al (2009) Prediction of functional outcome in acute cerebral hemorrhage using diffusion tensor imaging at 3T: a prospective study. AJNR Am J Neuroradiol 30(8):1561–1565. https://doi.org/10.3174/ajnr.A1639
Wang DM, Li J, Liu JR et al (2012) Diffusion tensor imaging predicts long-term motor functional outcome in patients with acute supratentorial intracranial hemorrhage. Cerebrovasc Dis 34(3):199–205. https://doi.org/10.1159/000341857
Kuzu Y, Inoue T, Kanbara Y et al (2012) Prediction of motor function outcome after intracerebral hemorrhage using fractional anisotropy calculated from diffusion tensor imaging. Cerebrovasc Dis 33(6):566–573. https://doi.org/10.1159/000338904
Tao WD, Wang J, Schlaug G et al (2014) A comparative study of fractional anisotropy measures and ICH score in predicting functional outcomes after intracerebral hemorrhage. Neurocrit Care 21(3):417–425. https://doi.org/10.1007/s12028-014-9999-2
Ma C, Liu A, Li Z et al (2014) Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. J Clin Neurosci 21(8):1388–1392. https://doi.org/10.1016/j.jocn.2013.11.032
Puig J, Blasco G, Terceño M et al (2019) Predicting motor outcome in acute intracerebral hemorrhage. AJNR Am J Neuroradiol 40(5):769–775. https://doi.org/10.3174/ajnr.A6038
Delcourt C, Sato S, Zhang S et al (2017) Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology 88(15):1408–1414. https://doi.org/10.1212/WNL.0000000000003771
Donnellan C, Werring D (2019) Cognitive impairment before and after intracerebral haemorrhage: a systematic review. Neurol Sci 1:148–219. https://doi.org/10.1007/s10072-019-04150-5
Poon MTC, Fonville AF, Al-Shahi Salman R (2014) Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 85(6):660–667. https://doi.org/10.1136/jnnp-2013-306476
Baykara E, Gesierich B, Adam R et al (2016) A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol 80(4):581–592. https://doi.org/10.1002/ana.24758
Funding
This work was undertaken at UCLH/UCL and received funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
Author information
Authors and Affiliations
Contributions
All co-authors contributed to the manuscript. SG, KB, PF, AG, GWKCAM and WDJ contributed to design of the study, analysis and interpretation of data, drafting the manuscript for intellectual content. BS, SR and JR contributed to major role in acquisition of data and revising manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
DJW has received honoraria from Bayer, Alnylam and NovoNordisk. RS received funding from UCLH/UCL BRC. The remaining authors declare no financial or other conflicts of interest.
Ethics approval
The SIGNaL registry (which contains routinely collected clinical data) is approved by the University College Hospitals NHS Foundation Trust Governance Review Board as a continuous service evaluation of a comprehensive clinical care program (service evaluation 5–201920-SE); for this reason, informed patient consent was not required.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwarz, G., Kanber, B., Prados, F. et al. Acute corticospinal tract diffusion tensor imaging predicts 6-month functional outcome after intracerebral haemorrhage. J Neurol 269, 6058–6066 (2022). https://doi.org/10.1007/s00415-022-11245-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11245-1