Abstract
Central and peripheral nervous system involvement during acute COVID-19 is well known. Although many patients report some subjective symptoms months after the infection, the exact incidence of neurological and cognitive sequelae of COVID-19 remains to be determined. The aim of this study is to investigate if objective neurological or cognitive impairment is detectable four months after SARS-CoV-2 infection, in a group of patients who had mild–moderate COVID-19. A cohort of 120 health care workers previously affected by COVID-19 was examined 4 months after the diagnosis by means of neurological and extensive cognitive evaluation and compared to a group of 30 health care workers who did not have COVID-19 and were similar for age and co morbidities. At 4 month follow-up, 118/120 COVID-19 cases had normal neurological examination, two patients had neurological deficits. COVID-19 patients did not show general cognitive impairment at MMSE. In COVID-19 cases the number of impaired neuropsychological tests was not significantly different from non COVID-19 cases (mean 1.69 and 1 respectively, Mann–Whitney p = n.s.), as well as all the mean tests’ scores. Anxiety, stress and depression scores resulted to be significantly higher in COVID-19 than in non COVID-19 cases. The results do not support the presence of neurological deficits or cognitive impairment in this selected population of mild–moderate COVID-19 patients four months after the diagnosis. Severe emotional disorders in patients who had COVID-19 in the past are confirmed.
Similar content being viewed by others
Introduction
COVID-19 has become a global public health problem [1]. SARS-CoV-2 principally targets the respiratory tract, causing potentially lethal bilateral interstitial pneumonia. Growing evidence, however, shows that COVID-19 can affect different organs and systems, including the central nervous system (CNS) [2, 3]. Notably, headache, nausea, vomiting, dizziness, myalgia, and fatigue are often reported during the acute disease, suggesting, together with the very frequent symptoms of anosmia and ageusia, a direct involvement of CNS and peripheral nervous system (PNS) [4,5,6].
In particular, an acute CNS involvement during SARS-CoV-2 infection has been reported, causing more often acute cerebrovascular diseases, conscious disturbances and delirium [7, 8]. Encephalomyelitis and peripheral neuropathies, i.e. Guillain Barrè radiculopathies or plexopaties have also been reported [9], emphasizing the need for a careful investigation of neurological signs in COVID-19 patients. Mechanisms of such involvement have been hypothesized: in addition to a direct viral invasion of neurons through trans synaptic transfer across infected neurons, entry via the olfactory nerve, infection of vascular endothelium, or leukocyte migration across the blood–brain barrier are considered [10]. Furthermore, hyperinflammation and hypercoagulability mechanisms are potential relevant etiological factors [10, 11].
Current estimates are that more than 20 million people globally have “recovered” from COVID-19; however, clinicians are observing and reading reports of patients with persistent severe symptoms and even substantial end-organ dysfunction after SARS-CoV-2 infection.
Because COVID-19 is a new infectious disease, much about the clinical course remains uncertain, in particular, the possible long-term health consequences, if any, and the impact of the severity of the disease on them. Interestingly, the persistence of several symptoms 60 days after the infection of SARS CoV2 has been reported in a recent series; these are, more frequently fatigue, dyspnea, joint pain, chest pain, caught, anosmia, sicca syndrome, rhinitis, red eyes, dysgeusia, headache, sputum production, lack of appetite, sore throat, vertigo, myalgia and diarrhea [12]. This points to a longstanding disease (the so called “long COVID”), rather than a self-limiting pathology. Interestingly, Huang et al. [13] reported in a group of patients from Wuhan, who were examined 6 months after the disease, that COVID-19 survivors were mainly troubled with fatigue or muscle weakness, sleep difficulties and anxiety or depression. Patients who were more severely ill during their hospital stay had more severely impaired diffusion capacities and abnormal chest imaging manifestations, resulting to be the main target population for intervention of long-term recovery. Morin et al. [14] also reported that of 478 survivors of severe COVID-19, who had been interviewed by telephone after 4 months, 31% of them reported fatigue, 21% cognitive symptoms and 16% dyspnea, that were not present before the disease. Of a subgroup of 177 patients (97 ICU and 80 non ICU) who were directly examined, the authors also observed an impairment in general cognition (MoCA score) or attention (d2-R score) in 38% of them. In addition to the fact that all these patients had had a severe COVID-19, other limitations of this study were the absence of an appropriate control group and the incomplete cognitive evaluation performed.
Studies of neurological post-acute sequelae of COVID-19 in patients who had a moderate or mild disease—which represents the majority of the cases—have not been published so far. Generally, longitudinal observational studies on distinct populations are critical to elucidate the health consequences attributable to COVID-19 and how these may compare with other serious illnesses [15].
The aim of the present study is to investigate the presence of neurological focal deficits as well as of cognitive impairment in a group of COVID-19 patients, who were examined 4 months after the diagnosis, from a population of health care workers (HCW) of a the hospital of Brescia (Italy).
Methods
The Unit of Occupational Health of the general University Hospital of Brescia, a Northern Italy town that has been hard hit by the SARS-CoV-2 pandemics, consecutively enrolled a group of HCW who had been previously affected by COVID-19 during the outbreak started in Italy by the end of February 2020. All of them had confirmed diagnosis of COVID-19, i.e. a positive result on a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab. The enrollment was conducted in the context of the mandatory occupational health surveillance program, to prospectively evaluate the health status after COVID-19. HCW underwent a targeted clinical diagnostic assessment including, among others, a neurological exam and a detailed neuropsychological evaluation including questionnaires. A written informed consent was obtained by each worker and the study followed the Ethics principles of the Helsinky Declaration. A total of 120 subjects of different categories (20 doctors, 71 physiotherapists, nurses or laboratory/ radiology technicians and 29 health care assistants) were examined. We included into the study a group of thirty HCW from the same Hospital who had not been previously affected by COVID-19.
Neurological and cognitive examination were conducted at the Neuropsychology Unit of the same Hospital: cranial nerve exam, strength, reflexes, sensory and coordination functions were assessed by a neurologist.
To comprehensively analyze all the cognitive functions that may have been involved by the disease, not limiting the analysis to a general cognition test, as MMSE [16], (which could possibly result not to be sensitive to catch subtle changes in cognitive abilities, particularly if the impairment is mild and selective), we preferred an extensive test battery usually used to investigate patients with brain diseases and specific for distinct cognitive functions, such as verbal and non-verbal memory, visuospatial and executive abilities, verbal fluency and attention [17]. All the tests results were corrected by age and education according to published Italian norms, to determine if their score was normal or impaired; the number of impaired neuropsychological tests was calculated for each subject, to have a measure of global impairment.
The following tests were used: Controlled Oral Word Association by categories (COWA) [18] for word fluency, Rey figure copy and recall [19] for visuospatial abilities and non-verbal memory, California Verbal Learning Test (CVLT) immediate and delayed recall [20] for verbal memory, TEA attention test [21] including visual reaction times (RT), auditory reaction times (RT), number of errors and of omissions for attention, Tower of London test (TOL) [22] for executive abilities. Finally MMSE [16] was considered as a measure of general cognitive impairment. Impairment in MMSE (score < 24) was considered as a measure of cognitive deterioration. Depression anxiety and stress scale-21 (DASS-21) [23] was used to measure emotional aspects, particularly related to stress, anxiety or depression. The study followed STROBE guidelines.
Statistics
The database was formatted through the Microsoft-Excel® software and later imported from the IBM-SPSS® software ver. 26.0.1 (IBM SPSS Inc. Chicago, Illinois). The use of the Stata® software ver. 16.0 (Stata Corporation, College Station, Texas) was also considered for comparisons or implementations of test output. Normality of the distributions was assessed using the Kolmogorov–Smirnov test. Categorical variables were presented as frequencies or percentages and compared with the use of the Chi-Square test or the Fisher’s exact test, as appropriate. Continuous variables were presented as means ± SD (in case of a normal distribution), or medians, and min/max (in case of a skewed distribution) and compared with the use of Student’s T-test, ANOVA, or the Mann–Whitney and Kruskal–Wallis test; correlations among variables by the Pearson’s or Spearman’s rank correlation test.
Analysis of covariance (ANCOVA) and univariate linear regression were also ran to study the relationships among dependent and independent variables.
A two-sided α level of 0.05 was used for all tests. The authors had full access to and take full responsibility for the integrity of the data.
Data availability statement
The authors are responsible for the correctness of the data and can share with other researchers the anonymized data set including all the data used, on request by qualified investigator.
Results
Demographic and clinical characteristics of COVID-19 and non COVID-19 subjects are shown in Table 1. Age and male/female ratio were not different between groups, as well as the frequency of the main comorbidities (hypertension, diabetes, obesity and respiratory diseases), though non COVID-19 subjects had a higher education than COVID-19 subjects (18 and 16 years of education respectively; p = 0.023), as doctors and biologists were significantly less frequent in non-COVID-19 as compared to COVID-19 HCW (43.3% vs 16.7%, respectively; p = 0.006). The other job titles (technicians, nurses, physiotherapists and health auxiliaries) were not differently represented between groups.
Most of the COVID-19 HCW had a mild–moderate disease, as 97.6% of them did not need oxygen therapy during the course of the infection. Only two of them had respiratory failure requiring hospitalization and oxygen therapy (in one case cPAP and in one intubation). Patients reported the following symptoms during the acute phase of the disease: fever, that was present in 91 of them (75.8%), ageusia in 78 (65%), anosmia in 77 (64.2%), asthenia in59 (49.2%), head pain in 47 (39.2%), caught in 39 (32.5%), diarrhea in 18 (15%). Interstitial pneumonia was diagnosed with chest radiography in 11 (9.2%), although during the March outbreak some pneumonia may have been underdiagnosed, as many of the patients self isolated at home and the availability of radiological exams was limited for a period.
Among those who exactly reported the used therapies, only 5% were treated with lopinavir/ritonavir combination, 16.7% with hydroxicloroquine and 5% with steroids. Antibiotics and anti-inflammatory drugs were also present in the medical history of home therapies at various extent.
The mean time between the first positive swab and the last negative one was 27 days (range 5–73 days). This time was arbitrarily considered as duration of the SARS-CoV-2 infection, as respiratory symptoms’ duration may not have been a correct measure of acute disease duration.
Time between the first positive nasopharyngeal swab and the neurological evaluation was 126 days, on average and was considered the follow-up duration.
At follow-up 118 out of 120 COVID-19 and all the non COVID-19 HCW had completely normal neurological exam. Two COVID-19 cases, showed areflexia and sensory loss with radicular distribution (L5 and S1 respectively) due to preexisting lumbar disc herniation.
At follow-up COVID-19 subjects still reported symptoms in 65% of cases (78/120): anosmia was the most frequently reported symptom (19% of cases), followed by fatigue, headache, attention difficulties, ageusia, dyspnea, joint and muscle pain, insomnia, memory difficulties, irritability and anxiety, hair loss, arrhythmia, hearing loss, tremors, dizziness, radicular pain and caught (Table 2).
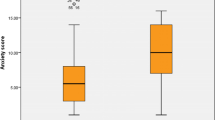
MMSE resulted to be normal in both patients and controls. Moreover the percentage of subjects with at least one impaired neuropsychological test or at least one impaired DASS-21 questionnaire was not statistically different between COVID-19 and non-COVID-19 subjects (Table 3). The mean number of impaired neuropsychological tests was 1.69 in COVID-19 and 1 in non COVID-19 subjects and this difference was not statistically significant. Notably, mean scores of all the neuropsychological tests were not statistically different between COVID-19 and non COVID-19 subjects (Table 3). The mean number of impaired DASS-21 questionnaires (1.83 in COVID-19 and 1.33 in non COVID-19 subjects) was not significantly different; on the other hand mean DASS-21 scores were all significantly higher in COVID-19 compared to non COVID-19 HCW (DASS-21depression score p = 0.036, DASS-21 stress score p = 0.013, DASS-21 anxiety scores p = 0.000). More frequently impaired cognitive tests were: TOL (15% COVID-19, 6.6% non-COVID-19), TEA omissions (8% COVID-19, 10% non COVID-19), Rey figure recall (8% COVID-19, 3.3% non COVID-19), Rey figure copy (5% COVID-19, 6.6% non COVID-19) and TEA auditory RT (5% COVID 19, 0% non COVID-19). The number of neuropsychological tests did not significantly differ between professional categories (Kruskall Wallis test p = n.s).
Scrutiny of selected cases with severe COVID-19 (a male doctor, aged 60 years, who needed cPAP and a male nurse, aged 50 years, who was intubated), did not reveal severe cognitive impairment in both of them: the first patient had normal results in all the cognitive tests and the second one was impaired in TOL only.
Although time from diagnosis (which was heterogeneous) was inversely correlated to the number of impaired neuropsychological tests (Spearman r = − 0.217; p = 0.018), suggesting that patients with less follow-up duration would show more impaired neuropsychological tests, linear regression analysis revealed that time from COVID-19 did not significantly influence the number of impaired tests. Furthermore, by linear regression, also education did not significantly influence the number of impaired tests or questionnaires and the number of symptoms reported at examination did not influence the number of impaired tests or questionnaires. Finally, the number of impaired neuropsychological tests, as well as DASS-21 scores did not significantly correlate with the duration of infection (Spearman test p = n.s).
On the other hand, DASS-21 anxiety, depression and stress questionnaires significantly influenced the majority of neuropsychological tests scores except TEA auditory RT and Rey figure recall (Table 4).
Discussion
The main finding of the present study is that in a selected population of patients, all belonging to the category of HCW, who were affected by COVID-19 during February 2020 outbreak, the frequency of neurological deficits and of cognitive impairment assessed four months later, were negligible. Patients showed impairment in 1.6 tests on average, which was not statistically different from the control group. Neither patients nor controls had cognitive deterioration at MMSE. Notably, patients’ scores in all the single tests used resulted to be not statistically different from those obtained by a control group of HCW of the same hospital, who did not have COVID-19. This finding was independent from education, type of work, duration of the infection and from the number of symptoms still reported at four month examination, which therefore was considerable (65% of cases) in COVID-19 subjects. On the other hand anxiety, stress and depression scores were significantly higher in COVID-19 compared with non COVID-19 subjects, suggesting a greater impact of COVID-19 on emotional wellbeing, rather than on cognition; not only in the acute phase of the disease, as already reported in the literature [24], but also in the long- term.
Although it is reasonable that the severity of the observed cognitive impairment may be greater in the initial stages following COVID-19, in our group time from diagnosis did not significantly influence it. It is possible that the absence of cognitive impairment in our sample could be due to the mild–moderate type of COVID 19 suffered by the majority of the cases; larger samples including patients with severe respiratory syndromes should be analyzed at the same follow-up length. Recently Morin et al. [14] reported an impairment in attention or in a test of general cognition in 42% of intubated patients 4 months after COVID-19, but the pathogenesis of such an impairment could be related to either a direct effect of SARS-CoV2 or to a general effect of intensive care, as reported in other patients after critical illness [25]. Nevertheless the cause of such an impairment may be multi factorial, as metabolic deficits or factors related to coagulopathies or hypoxia are implicated and the clinical phenotypes of ICU patients should be separately analyzed, to identify the precise pathogenesis in individual cases. Further investigations, on larger samples including imaging and laboratory studies will be needed in the future.
Our findings add relevant information about the clinical evolution of COVID-19 over time, with particular reference to the implications of SARS-CoV2 infection on cognition. The well known involvement of CNS and PNS in the acute phase of the disease has already been reported in the literature [10] and the biological pathways that could underlie each neurological complication of COVID-19 have been hypothesized [3]. Although some authors failed to establish insights into nervous system manifestations of COVID-19 in systematic reviews [26], and long-term neurological consequences, if any, are unknown.
Basing on our study, at least in this population of predominantly mild/moderate cases of COVID-19 patients, neurological examination is substantially normal and cognition is not significantly worse compared to that well matched for sociodemographic characteristics controls, at four month follow-up. Specifically, neither general cognitive deterioration nor deficits in selective cognitive functions was detected. This does not support the concept of a persistent viral induced brain damage. Even if a broad organotropism of SARS-CoV-2 has been reported in autopsies of patients deceased with COVID-19 [27], SARS-CoV-2 has not been detected in cerebrospinal fluid of the majority of individuals with COVID-19 and encephalopathies so far [28] and a direct viral invasion of SARS-CoV-2 in neuropathological studies has not been clearly demonstrated [3]. Overall, the lack of typical neuropathological features of viral and post viral encephalitis in acute COVID-19 patients with neurological deficits argues against the hypothesis of direct damaging effects on the CNS and our results are in line with these observations.
The main limitation of this research is that the studied sample is mainly made of mild–moderate COVID-19 cases, who, in the majority of cases, did not need oxygen therapy. On the other hand, this phenotype reflects the epidemiology of COVID-19. Though, the two cases in our series, who had a more severe illness and needed oxygen therapy, did not show cognitive impairment. Furthermore, unfortunately we did not have any cognitive evaluation of HCW before COVID-19 that could possibly show small differences from baseline in patients.
In our study stress, depression and anxiety appear to be significantly worse in COVID-19 subjects compared to the control population of HCW, who were similarly exposed to the same stressful pandemic experience in the hospital and to the same lockdown experience at home. This suggests that the disease itself may have direct consequences on mood and anxiety, as already described in MERS survivors, who, in 25% of cases, showed signs of post-traumatic stress disorder, whereas 15.6% of them had worsening depression [29]. Certainly, the COVID-19 pandemic has highlighted the urgent need to address the emotional wellbeing of clinicians, particularly if they were affected by COVID-19. Programs should be designed with a range of strategies, including psychological support and resources for mitigating workplace stressors [30].
References
Bulut C, Kato Y (2020) Epidemiology of COVID-19. Turk J Med Sci 50:563–570. https://doi.org/10.3906/sag-2004-172
Romero-Sánchez CM et al (2020) Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. https://doi.org/10.1212/WNL.0000000000009937
Pezzini A, Padovani A (2020) Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol 16:636–664
Li H, Xue Q, Xu X (2020) Involvement of the nervous system in SARS-CoV-2 infection. Neurotoxicity Res. https://doi.org/10.1007/s12640-020-00219-8
Politi LS, Salsano E, Grimaldi M (2020) Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. Images Neurol. https://doi.org/10.1001/jamaneurol.2020.2125
Correira AO, FeitosaPW G, De Sousa MJL, Nogueira SAR, Fonseca RB, Pereira Nobre ME (2020) Neurological manifestation of COVID-19 and other coronaviruses: a systematic review. Neurol Psychiatry Brain Res. https://doi.org/10.1016/j.npbr.2020.05.008
Helms J, Kremer S, Merdji H et al (2020) Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. https://doi.org/10.1056/NEJMc2008597
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1127
Paterson RW, Brown RL, Benjamin L et al (2020) The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 143(10):3104–3120. https://doi.org/10.1093/brain/awaa240
Zubair AS, McAlpine LS, Gardin T et al (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019. A review. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.2065
Alberti P, Beretta S, Piatti S et al (2020) (2020) Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm 7:e741. https://doi.org/10.1212/NXI.0000000000000741
Carfì A, Bernabei R, Landi F, Gemelli against COVID-19 Post-Acute Care Study Group (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–605. https://doi.org/10.1001/jama.2020
Huang C, Huang L, Wang Y et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397:220–232. https://doi.org/10.1016/S0140-6736(20)32656-8
Morin et al. The Writing Committee for the COMEBAC Study Group (2021) Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. https://doi.org/10.1001/jama.2021.3331
Del Rio C, Collins MF, Malani P (2020) Long-term health Consequences of COVID-19. JAMA. https://doi.org/10.1001/jama.2020.19719
Folstein MF, Robins LN, Helzer JE (1983) The mini-mental state examination. Archiv Gen Psychiatry 40(7):812–812
Lezak MD (1995) Neuropsychological assessment, 3rd edn. Oxford University Press, Oxford
Novelli G, Papagno C, Capitani E, Laiacona M (1986). Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. Archivio di Psicol Neurol Psichiatr
Caffara P, Vezzadini G et al (2002) Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22:443–447
Delis DC, Kramer JH, Kaplan E, Ober BA (2000) California verbal learning test, 2nd edition (CVLT-II). Psychological Corporation, San Antonio, TX
Zimmermann P, Fimm B (1997) Batteria di Test per l'esame dell'attenzione (TEA). Italian Edition by Pizzamiglio L, Zoccolotti P, Pittau PA
Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L (2004) The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 68(2–3):283–297
Lovibond SH, Lovibond PF (1995) Manual for the depression anxiety & stress scales, 2nd edn. Psychology Foundation, Sydney
Zhang W, Wang K, Yin L, Zhao W, Xue Q et al (2020) Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychotherapy Psychosomat. https://doi.org/10.1159/000507639
Pandharipande PP, Girard TD, Jackson JC et al (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369:1306–1316
Romoli M, Jelcic I, Bernard Vallet R (2020) A systematic review of neurological manifestations of SARS-CoV-2 infection: the devil is hidden in the details. Eur J Neurol 27:712–726. https://doi.org/10.1111/ene.14382
Puelles VG, Lütgehetmann M, Lindenmeyer MT et al (2020) Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 383(6):590–592
Schaller T, Hirschbuhl K, Burkhardt K et al (2020) Postmortem examination of patients with COVID-19. JAMA 323(24):2518–2520. https://doi.org/10.1001/jama.2020.8907
Mak IW, Chu CM, Pan PC, Yiu MG, Chan VL (2009) Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry 31:318–326. https://doi.org/10.1016/j.genhosppsych.2009.03.001
Shapiro J, McDonald T (2020) Supporting clinicians during Covid-19 and beyond learning from past failures and envisioning new strategies. New Engl J Med
Acknowledgements
The authors wish to thank Anna Maria Indelicato (MD) for support in organization, Giulia Paderni and Jasmine Rivolta (Psychologists) for supporting the conduction of the study and for helping in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicting interests.
Rights and permissions
About this article
Cite this article
Mattioli, F., Stampatori, C., Righetti, F. et al. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J Neurol 268, 4422–4428 (2021). https://doi.org/10.1007/s00415-021-10579-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10579-6