Abstract
Objectives
To investigate the association between cognitive function at baseline and the progression of motor disability in Parkinson’s disease (PD).
Methods
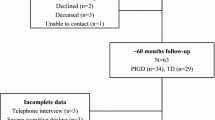
We consecutively enrolled 257 drug-naïve patients with early-stage PD (follow-up > 2 years) who underwent a detailed neuropsychological test at initial assessment. Factor analysis was conducted to yield four cognitive function factors and composite scores thereof: Factor 1 (visual memory/visuospatial), Factor 2 (verbal memory), Factor 3 (frontal/executive), and Factor 4 (attention/working memory/language). The global cognitive composite score of each patient was calculated based on these factors. Subsequently, we assessed the effect of baseline cognitive function on long-term motor outcomes, namely levodopa-induced dyskinesia (LID), wearing-off, freezing of gait (FOG), and rate of longitudinal increases in levodopa-equivalent dose (LED).
Results
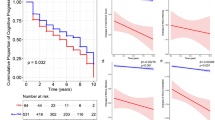
Cox regression analysis demonstrated that higher Factor 3 (frontal/executive) composite scores (i.e., better cognitive performance) were associated with early development of LID [hazard ratio (HR), 1.507; p = 0.003], whereas higher Factor 1 (visual memory/visuospatial) composite scores (i.e., better cognitive performance) were associated with a lower risk for FOG (HR 0.683; p = 0.017). We noted that higher global cognitive composite scores were associated with a lower risk for developing FOG (HR 0.455; p = 0.045). The linear mixed model demonstrated that higher global cognitive composite scores and better cognitive performance in visual memory/visuospatial function were associated with slower longitudinal increases in LED.
Conclusions
These findings suggest that baseline cognitive profiles have prognostic implications on several motor aspects in patients with PD.

Similar content being viewed by others
References
Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65:1239–1245
Pedersen KF, Larsen JP, Tysnes OB, Alves G (2013) Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 70:580–586
Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G (2010) What predicts mortality in Parkinson disease? A prospective population-based long-term study. Neurology 75:1270–1276
Marras C, Rochon P, Lang AE (2002) Predicting motor decline and disability in Parkinson disease: a systematic review. Arch Neurol 59:1724–1728
Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP (2005) Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology 65:1436–1441
Velseboer DC, Broeders M, Post B, van Geloven N, Speelman JD, Schmand B, de Haan RJ, de Bie RM (2013) Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology 80:627–633
Burn DJ, Rowan EN, Allan LM, Molloy S, O’Brien JT, McKeith IG (2006) Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 77:585–589
Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D (2006) Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 21:1123–1130
Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, Auinger P, Chou KL, Growdon JC (2009) Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 73:1469–1477
Ray NJ, Strafella AP (2012) The neurobiology and neural circuitry of cognitive changes in Parkinson’s disease revealed by functional neuroimaging. Mov Disord 27:1484–1492
McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D (2010) Characteristics of executive function impairment in Parkinson’s disease patients without dementia. J Int Neuropsychol Soc 16:268–277
Pillon B, Deweer B, Agid Y, Dubois B (1993) Explicit memory in Alzheimer’s, Huntington’s, and Parkinson’s diseases. Arch Neurol 50:374–379
Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP (2003) Performance on the dementia rating scale in Parkinson’s disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 74:1215–1220
Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA (2009) The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain 132:2958–2969
van der Zee S, Müller M, Kanel P, van Laar T, Bohnen NI (2020) Cholinergic denervation patterns across cognitive domains in Parkinson’s disease. Mov Disord 36:642–650
Coelho M, Ferreira JJ (2012) Late-stage Parkinson disease. Nat Rev Neurol 8:435–442
Chung SJ, Yoo HS, Oh JS, Kim JS, Ye BS, Sohn YH, Lee PH (2018) Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Parkinsonism Relat Disord 51:43–48
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Kang Y (2012) Seoul Neuropsychological Screening Battery (SNSB-II). Human Brain Research and Consulting Co., Seoul
Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 28:668–670
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Chung SJ, Yoo HS, Lee HS, Oh JS, Kim JS, Sohn YH, Lee PH (2018) The pattern of striatal dopamine depletion as a prognostic marker in de novo Parkinson disease. Clin Nucl Med 43:787–792
Chung SJ, Lee Y, Oh JS, Kim JS, Lee PH, Sohn YH (2018) Putaminal dopamine depletion in de novo Parkinson’s disease predicts future development of wearing-off. Parkinsonism Relat Disord 53:96–100
Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N (2003) Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol 10:391–398
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Chung SJ, Lee HS, Kim HR, Yoo HS, Lee YH, Jung JH, Baik K, Ye BS, Sohn YH, Lee PH (2020) Factor analysis-derived cognitive profile predicting early dementia conversion in PD. Neurology 95:e1650–e1659
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Yoo HS, Chung SJ, Lee PH, Sohn YH, Kang SY (2019) The influence of body mass index at diagnosis on cognitive decline in Parkinson’s disease. J Clin Neurol 15:517–526
Kang SJ (2002) The reliability and validity of the Korean instrumental activities of daily living (K-IADL). J Korean Neurol Assoc 20:8–14
Ku HM, Kim JH, Kwon EJ, Kim SH, Lee HS, Ko HJ, Jo S, Kim DK (2004) A study on the reliability and validity of seoul-instrumental activities of daily living (S-IADL). J Korean Neuropsychiatr Assoc 43:189–199
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Hair JF, Black WC, Babin BJ, Anderson RE (2013) Multivariate data analysis. Pearson Education Limited
Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G (2015) A 12-year population-based study of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 21:254–258
Sunwoo MK, Hong JY, Lee JJ, Lee PH, Sohn YH (2016) Does education modify motor compensation in Parkinson’s disease? J Neurol Sci 362:118–120
Kotagal V, Bohnen NI, Muller ML, Koeppe RA, Frey KA, Langa KM, Albin RL (2015) Educational attainment and motor burden in Parkinson’s disease. Mov Disord 30:1143–1147
Blume J, Rothenfusser E, Schlaier J, Bogdahn U, Lange M (2017) Educational attainment and motor burden in advanced Parkinson’s disease—the emerging role of education in motor reserve. J Neurol Sci 381:141–143
Lee PC, Artaud F, Cormier-Dequaire F, Rascol O, Durif F, Derkinderen P, Marques AR, Bourdain F, Brandel JP, Pico F, Lacomblez L, Bonnet C, Brefel-Courbon C, Ory-Magne F, Grabli D, Klebe S, Mangone G, You H, Mesnage V, Brice A, Vidailhet M, Corvol JC, Elbaz A (2019) Examining the reserve hypothesis in Parkinson’s disease: a longitudinal study. Mov Disord 34:1663–1671
Yogev-Seligmann G, Hausdorff JM, Giladi N (2008) The role of executive function and attention in gait. Mov Disord 23:329–342
Hausdorff JM, Buchman AS (2013) What links gait speed and MCI with dementia? A fresh look at the association between motor and cognitive function. J Gerontol A Biol Sci Med Sci 68:409–411
Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM (2012) Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 60:2127–2136
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Thenganatt MA, Jankovic J (2014) Parkinson disease subtypes. JAMA Neurol 71:499–504
Rascol O, Sabatini U, Brefel C, Fabre N, Rai S, Senard JM, Celsis P, Viallard G, Montastruc JL, Chollet F (1998) Cortical motor overactivation in parkinsonian patients with L-dopa-induced peak-dose dyskinesia. Brain 121(Pt 3):527–533
Cerasa A, Pugliese P, Messina D, Morelli M, Gioia MC, Salsone M, Novellino F, Nicoletti G, Arabia G, Quattrone A (2012) Prefrontal alterations in Parkinson’s disease with levodopa-induced dyskinesia during fMRI motor task. Mov Disord 27:364–371
Cerasa A, Messina D, Pugliese P, Morelli M, Lanza P, Salsone M, Novellino F, Nicoletti G, Arabia G, Quattrone A (2011) Increased prefrontal volume in PD with levodopa-induced dyskinesias: a voxel-based morphometry study. Mov Disord 26:807–812
Cerasa A, Morelli M, Augimeri A, Salsone M, Novellino F, Gioia MC, Arabia G, Quattrone A (2013) Prefrontal thickening in PD with levodopa-induced dyskinesias: new evidence from cortical thickness measurement. Parkinsonism Relat Disord 19:123–125
Aron AR, Obeso J (2012) Is executive control used to compensate for involuntary movements in levodopa-induced dyskinesia? Mov Disord 27:339–340
Yoo HS, Chung SJ, Lee YH, Lee HS, Ye BS, Sohn YH, Lee PH (2019) Levodopa-induced dyskinesia is closely linked to progression of frontal dysfunction in PD. Neurology 92:e1468–e1478
Jenner P (2013) Wearing off, dyskinesia, and the use of continuous drug delivery in Parkinson’s disease. Neurol Clin 31:S17-35
de la Fuente-Fernandez R, Schulzer M, Mak E, Calne DB, Stoessl AJ (2004) Presynaptic mechanisms of motor fluctuations in Parkinson’s disease: a probabilistic model. Brain 127:888–899
Boyce S, Rupniak NM, Steventon MJ, Iversen SD (1990) Nigrostriatal damage is required for induction of dyskinesias by L-DOPA in squirrel monkeys. Clin Neuropharmacol 13:448–458
Amboni M, Cozzolino A, Longo K, Picillo M, Barone P (2008) Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord 23:395–400
Teramoto H, Morita A, Ninomiya S, Shiota H, Kamei S (2014) Relation between freezing of gait and frontal function in Parkinson’s disease. Parkinsonism Relat Disord 20:1046–1049
Sunwoo MK, Cho KH, Hong JY, Lee JE, Sohn YH, Lee PH (2013) Thalamic volume and related visual recognition are associated with freezing of gait in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord 19:1106–1109
Tard C, Delval A, Duhamel A, Moreau C, Devos D, Dujardin K (2015) Specific attentional disorders and freezing of gait in Parkinson’s disease. J Parkinsons Dis 5:379–387
Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A (2019) Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 63:77–82
Domellof ME, Elgh E, Forsgren L (2011) The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson’s disease. Mov Disord 26:2183–2189
Domellof ME, Forsgren L, Elgh E (2013) Persistence of associations between cognitive impairment and motor dysfunction in the early phase of Parkinson’s disease. J Neurol 260:2228–2236
Nantel J, McDonald JC, Tan S, Bronte-Stewart H (2012) Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson’s disease. Neuroscience 221:151–156
Vercruysse S, Devos H, Munks L, Spildooren J, Vandenbossche J, Vandenberghe W, Nieuwboer A, Heremans E (2012) Explaining freezing of gait in Parkinson’s disease: motor and cognitive determinants. Mov Disord 27:1644–1651
Helmich RC, de Lange FP, Bloem BR, Toni I (2007) Cerebral compensation during motor imagery in Parkinson’s disease. Neuropsychologia 45:2201–2215
Lewis GN, Byblow WD, Walt SE (2000) Stride length regulation in Parkinson’s disease: the use of extrinsic, visual cues. Brain 123(Pt 10):2077–2090
McColl CD, Reardon KA, Shiff M, Kempster PA (2002) Motor response to levodopa and the evolution of motor fluctuations in the first decade of treatment of Parkinson’s disease. Mov Disord 17:1227–1234
Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD (2005) Visual dysfunction in Parkinson disease without dementia. Neurology 65:1907–1913
Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K (1999) Progression of parkinsonian signs in Parkinson disease. Arch Neurol 56:334–337
von Bonsdorff M, Rantanen T, Laukkanen P, Suutama T, Heikkinen E (2006) Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology 52:359–365
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2019R1A2C2085462) and the Ministry of Education (NRF-2018R1D1A1B07048959).
Author information
Authors and Affiliations
Contributions
SJC and PHL conceived the study. SJC, HSY, HSL, YHL, KWB, and JHJ acquired and analyzed the data. SJC and PHL wrote the manuscript. HSY, HSL, YHL, KWB, JHJ, BSY, and YHS revised the manuscript for important intellectual content. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no financial or other conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of Yonsei University Severance Hospital. The need for informed consent was waived because of the retrospective nature of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chung, S.J., Yoo, H.S., Lee, H.S. et al. Baseline cognitive profile is closely associated with long-term motor prognosis in newly diagnosed Parkinson’s disease. J Neurol 268, 4203–4212 (2021). https://doi.org/10.1007/s00415-021-10529-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10529-2