Abstract
Background
Little is known about newly developed stroke in patients admitted to the intensive care unit (ICU).
Objective
This study aimed to investigate characteristics and outcomes of newly developed stroke in patients admitted to the non-neurological intensive care units (ICU-onset stroke, IOS).
Methods
A consecutive series of adult patients who were admitted to the non-neurological ICU were included in this study. We compared neurological profiles, risk factors, and mortality rates between patients with IOS and those without IOS.
Results
Of 18,604 patients admitted to the ICU for non-neurological illness, 218 (1.2%) developed stroke (ischemic, n = 182; hemorrhagic, n = 36). The most common neurological presentation was altered mental status (n = 149), followed by hemiparesis (n = 55), and seizures (n = 28). The most common etiology of IOS was cardioembolism (50% [91/182]) for ischemic IOS and coagulopathy (67% [24/36]) for hemorrhagic IOS. In multivariable analysis, the Acute Physiology and Chronic Health Evaluation II (APACHE II) score (adjusted odds ratio [AOR] = 1.04, 95% CI = 1.03−1.06, P < 0.001), prothrombin time (AOR = 0.99, 95% CI = 0.98−0.99, P = 0.013), cardiovascular surgery (AOR = 1.84, 95% CI = 1.34−2.50, P < 0.001), mechanical ventilation (AOR = 6.75, 95% CI = 4.87−9.45, P < 0.001), and extracorporeal membrane oxygenation (AOR = 2.77, 95% CI = 1.62−4.55, P < 0.001) were related to the development of IOS. Stroke was associated with increased 3-month mortality after hospital discharge (AOR, 2.20; 95% CI, 1.58–3.05; P < 0.001), after adjustment for APACHE II and comorbidities.
Conclusions
Patients who developed IOS had characteristics of initial critical illness and managements performed in the ICU as well as neurological presentations. The occurrence of IOS was related to high morbidity and mortality.
Similar content being viewed by others
Introduction
In conjunction with high incidence, in-hospital stroke showed lower rates in reperfusion therapy and greater risk of mortality compared with community-onset stroke [1,2,3]. Patients admitted to intensive care unit (ICU) have distinct features such as unstable vital signs, coagulopathies, inflammation, as well as multiple comorbidities, and they often receive invasive procedures or surgical treatments. Thus, there may be high risks of stroke as well as delays in the diagnosis of stroke during admission to the ICU, leading to suboptimal management of critically ill patients [4, 5].
Early detection of acute stroke is imperative for saving viable brain tissues and recovery of neurologic deficits [6]. Time saving measures are being implemented at every step from symptom recognition, imaging studies, and treatments. However, early detection of stroke symptoms in ICU-onset stroke (IOS) is challenging, not only due to comorbidities, but also due to immobilization, medical equipment, and use of sedative agents [7, 8]. Accordingly, there are substantial barriers to the conduct of neuroimaging studies to reveal IOS in the context of general critical care. Comprehensive studies regarding IOS are lacking, which limit the development of strategies for the prevention, early detection, and proper managements of IOS.
Here, we aimed to investigate the neurological and radiological profiles, risk factors, and clinical outcomes of IOS. Furthermore, we aimed to investigate differences of such characteristics between ischemic and hemorrhagic strokes.
Methods
Study population
This study was performed at Asan Medical Center, a 2700-bed tertiary hospital in Seoul, Republic of Korea. For this study, medical records of a consecutive series of ICU patients between November 1st, 2012 and March 31st, 2016 were retrospectively evaluated. We included patients who (1) were 18 years of age or older, (2) were admitted to clinical departments other than neurology and neurosurgery, and (3) did not have acute stroke before ICU admission. This study was approved by the institutional review board of Asan Medical Center, and the need for written informed consent was waived because of the retrospective design of the study.
Routine evaluations in the intensive care units
According to the routine practice of our ICUs, neurological evaluations, including the Glasgow Coma Scale, a pupillary size, light reflexes, and muscle strength (the Medical Research Council scale), were performed and documented by nurses every 1−2 h. When nurses detected abnormal neurological findings during their routine evaluations, they notified such findings to treating doctors perform computed tomography (CT) or magnetic resonance imaging (MRI) scans of the brain.
We reviewed electronic medical records for patients’ baseline characteristics, laboratory findings, and Acute Physiology and Chronic Health Evaluation II (APACHE II) score at the time of admission to the ICUs [9]. We investigated whether the patients underwent surgery (cardiovascular vs. non-cardiovascular surgery) and invasive cardiovascular interventions before the occurrence of stroke. Moreover, we assessed the application of life-support modalities such as inotropic agents, mechanical ventilation, continuous renal replacement therapy, or extracorporeal membrane oxygenation (ECMO). Systemic inflammatory response syndrome (SIRS) was defined in accordance with international guidelines [10].
Definition of intensive care unit-onset stroke
We defined IOS if (1) CT and/or MRI scans of the brain were performed during ICU admission and (2) CT and/or MRI images revealed findings compatible with acute infarcts, intracerebral hemorrhage (ICH), or subarachnoid hemorrhage (SAH). We dichotomized IOS into ischemic IOS (infarcts) and hemorrhagic IOS (ICH and SAH).
Neurological and radiological assessments
For patients with a diagnosis of IOS, we categorized their symptoms (or signs) into categories such as altered mental status, seizures, pupillary changes (size and light reflexes), hemiparesis, and others. Time domains such as last-known-normal time, first-found-abnormal time, and time to initial neuroimaging studies were also reviewed. Clinical outcomes included length of ICU stay, length of hospital stay, mortality before ICU discharge, mortality before hospital discharge, mortality at 90 days after hospital discharge, and mortality at 90 days from ICU admission.
We evaluated for the presence of vascular stenosis (> 50% reduction of vascular diameter) or occlusion, if the patient underwent cerebral angiography. The subtypes of ischemic IOS were determined according to the classification of the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) [11]. For patients with ICH, we measured the ICH score as well as the lesion location and volume [12]. We categorized the etiologies of ICH into hypertension, cerebral amyloid angiopathy, coagulopathy, medication (antiplatelet agents or anticoagulants), and unknown cause groups [13]. For patients with SAH, we reviewed CT brain scans to identify the presence of ruptured aneurysms and assessed SAH severity using the modified Fisher scale. Neuroimaging studies (CT, MRI, and angiographic studies) were reviewed jointly by two investigators and a third investigator was consulted in case of disagreements.
Clinical management
Treatment modalities for ischemic IOS included antiplatelet agents, anticoagulants, intravenous thrombolysis, intraarterial thrombectomy, and neurosurgery, and those for hemorrhagic IOS were categorized into either neurosurgical or medical treatments.
Statistical analysis
We compared baseline demographics, comorbidities, APACHE II score, the presence of SIRS, laboratory findings, and treatment modalities between patients with IOS and those without IOS using χ2 tests, t tests, and Kruskal–Wallis tests, as appropriate. Variables with a P value of < 0.2 by univariate analysis were included as candidate variables in multivariable analysis. Backward stepwise selection was conducted to find factors associated with IOS in multivariable logistic regression models. We further performed all analyses using a forward selection procedure to confirm the final model. We also compared the aforementioned variables between patients with ischemic IOS and those with hemorrhagic IOS. A Cox proportional hazards model was used to assess the hazard ratios of 90-day mortality from ICU admission according to the presence of stroke, with adjustments for demographics, comorbidities, and APACHE II scores. Kaplan–Meier survival curves were also plotted for mortality of patients with IOS and patients without IOS. Additionally, we evaluated the association between stroke and mortality, with adjustments for demographics, comorbidities, and APACHE II scores using multivariate logistic regression. All statistical analyses were performed using R, version 3.4.3 (R Foundation for Statistical Computing, Vienna. Austria) and SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 27,679 patients were admitted to 8 adult ICUs during the study period. Of these, we excluded patients younger than 18 years of age (n = 514), those who were admitted to neurological and neurosurgical departments (n = 8123), and patients diagnosed with acute stroke before admission to the ICU (n = 438). Thus, we finally included 18,604 patients. The median age of included patients was 63.0 years (IQR, 53.0−72.0 years) and 11,993 (64.5%) patients were male. Table 1 shows baseline characteristics of the finally included 18,604 patients.
Intensive care unit-onset stroke assessments
The incidence of IOS in our study population was 1.2% (218/18,604). Of 18,604 patients, 4056 (21.8%) underwent CT/MRI brain scans (CT, n = 824 [4.4%]; MRI, n = 2552 [13.7%]; and CT + MRI, n = 680 [3.7%]). Of the 4056 patients who underwent CT/MRI imaging, 218 had findings compatible with acute stroke; of these, 182 (83.5%) had ischemic IOS, and 36 (16.5%) had hemorrhagic IOS (ICH, n = 33; and SAH, n = 3).
Altered mental status (n = 149) was the most common neurological manifestation of IOS (the reason to conduct neuroimaging studies), followed by hemiparesis (n = 55), seizures (n = 28), pupillary changes (n = 16), and aphasia (n = 9). Comatose state (52.8% vs. 13.7%; p < 0.001) and pupillary changes (25.0% vs. 3.8%; p < 0.001) were more common in patients with hemorrhagic IOS than those with ischemic IOS, while aphasia (4.9% vs. 0%; p < 0.001) and hemiparesis (29.7% vs. 2.8%; p < 0.001) were more common in patients with ischemic IOS than those with hemorrhagic IOS. (Table I in Data Supplement).
When we compared ischemic IOS with hemorrhagic IOS, there was no significant difference in the time interval for stroke recognition (9.1 h [IQR, 2.3−23.5 h] vs. 8.4 h [IQR, 4.1−29.9 h]; p = 0.482). Of 218 patients with IOS, the median time interval from the first-found-abnormal time to the first neuroimaging studies (CT or MRI scans of the brain), i.e., time interval for neuroimaging, was 5.4 h (IQR, 1.5−24.5 h); the median time interval for neuroimaging studies was significantly longer for patients with ischemic IOS compared to patients with hemorrhagic IOS (182 patients with ischemic IOS, 6.7 h [IQR, 1.9−29.3 h]; 36 patients with hemorrhagic IOS, 1.8 h [IQR, 1.0−7.4 h); p = 0.010).
The main reasons for delays in stroke recognition included the use of sedative agents following surgery (n = 51) or mechanical ventilation (n = 29), presumed metabolic encephalopathy (n = 18), and missed findings of neurological deficits during routine hourly evaluations (n = 4) (as described for 102 patients who had such a time interval beyond the median time of 8.9 h). Patients with altered mental status as an initial stroke manifestation had time delays to the stroke recognition than patients without altered mental status (p = 0.027), while patients with seizure as an initial stroke manifestation had shorter time intervals for the stroke recognition than patients without seizure (p = 0.047). The main reasons for delays in neuroimaging study included unstable vital signs (n = 22), the application of ECMO (n = 8), poor cooperation of patients (n = 3), and unknown reasons (n = 70) (as described for 103 patients who had a time interval beyond the median time of 5.4 h). Patients with hemiparesis or pupillary changes as a stroke manifestation had shorter time intervals for neuroimaging studies than patients without those symptoms (p = 0.002 and 0.006, respectively) (Table 2).
Radiological findings and presumed etiologies of ischemic IOS and hemorrhagic IOS are shown in Table 3. Of the 182 patients with ischemic IOS, cardioembolism (50.0%) was the most common etiology of ischemic IOS, followed by undetermined etiology (39.6%), other determined etiology (6.0%), large-artery disease (2.7%), and small-vessel disease (1.6%). Other determined etiologies included cancer-related stroke (n = 5), cerebral air embolism (n = 3), and arterial dissection (n = 2), and meningitis-related stroke (n = 1). Of the 33 patients with ICH, the most common etiology of ICH was coagulopathy (n = 24), and the presumed causes of such coagulopathy were liver disease (n = 11), sepsis (n = 9), and hematologic malignancy (n = 4). Among 3 patients with SAH, modified Fisher scale was 4 in 2 patients and 1 in 1 patient, and only 1 patient had a ruptured aneurysm.
Risk factors of intensive care unit-onset stroke
In the univariable analysis, risk factors associated with IOS were older age, APACHE II score, SIRS, cardiovascular surgery, non-cardiovascular surgery, use of mechanical ventilation, continuous renal replacement therapy, and use of ECMO. The following laboratory findings were also associated with IOS: hemoglobin level, platelet count, and prothrombin time (p < 0.001 for all variables). Multivariable analysis indicated that APACHE II score (adjusted odds ratio [AOR] = 1.04; 95% CI = 1.03−1.06; p < 0.001), prothrombin time (AOR = 0.99; 95% CI = 0.98−0.99; p = 0.013), cardiovascular surgery (AOR = 1.84; 95% CI = 1.34−2.50; p < 0.001), use of mechanical ventilation (AOR = 6.75; 95% CI = 4.87−9.45; p < 0.001), and use of ECMO (AOR = 2.77; 95% CI = 1.62−4.55; p < 0.001) were related to the occurrence of IOS. When patients with ischemic IOS were compared with patients without IOS, factors associated with ischemic IOS were APACHE II score (AOR = 1.05; 95% CI = 1.03−1.06; p < 0.001), cardiovascular surgery (AOR = 1.84; 95% CI = 1.34–2.50; p < 0.001), use of mechanical ventilation (AOR = 6.56; 95% CI = 4.64−9.40; p < 0.001), and use of ECMO (AOR = 2.72; 95% CI = 1.47−4.73; p = 0.001). When patients with hemorrhagic IOS were compared with patients without IOS, factors associated with hemorrhagic IOS were APACHE II score (AOR = 1.04; 95% CI = 1.01−1.07; p = 0.014), prothrombin time (AOR = 0.98; 95% CI = 0.97−0.99; p = 0.002), use of mechanical ventilation (AOR = 10.04; 95% CI = 4.16−28.10; p < 0.001), and use of ECMO (AOR = 3.71; 95% CI = 1.32−9.01; p = 0.007) (Table 4).
Managements for intensive care unit-onset stroke
Of the 182 patients with ischemic IOS, antithrombotic agents (antiplatelet agents and anticoagulants) were given to 164 patients. Antithrombotic agents were not given to 18 patients due to thrombocytopenia (n = 13), hemoptysis (n = 2), large infarct size (n = 2), and for uncertain reasons (n = 1). The reperfusion therapy rate for IOS was 7.1% (13/182; intravenous thrombolysis, n = 5; intraarterial thrombectomy, n = 8) in our study population. Intravenous thrombolysis (infusion of alteplase) was attempted in 5 patients. The reasons not to perform intravenous thrombolysis in the remaining 177 patients were as follows: neuroimaging studies were performed beyond 4.5 h from the last-known-normal time (n = 159), patients underwent recent major surgery (n = 9), had large hemispheric infarct (n = 1), prolonged activated partial thromboplastin time (n = 1), mild neurological deficits (n = 1), delays in decision-making (n = 2), and uncertain reasons (n = 4). Of 18 patients with large-artery occlusion, intraarterial thrombectomy was performed in 8 patients. Of the remaining 10 patients, intraarterial thrombectomy was not attempted due to reasons such as the patients having absence of diffusion-perfusion mismatch (n = 4), recent aortic surgeries (n = 3), rapidly resolving neurological symptoms (n = 1), unstable vital signs (n = 1), or for uncertain reasons (n = 1). Two patients received decompressive craniectomy for large hemispheric infarcts. Of the 36 patients with hemorrhagic IOS, 5 (13.9%) underwent surgical treatments including decompressive hemicraniectomy (n = 3), decompressive hemicraniectomy with hematoma evacuation (n = 1), and bilateral frontotemporal decompressive craniectomy (n = 1).
Clinical outcomes of patients with ICU-onset stroke
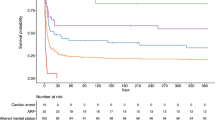
The length of ICU stay was longer in patients with IOS compared with those without IOS (median days, 12.0 [IQR, 5.0–26.5] vs. 2.0 [IQR, 1.0−4.0]; p < 0.001). Accordingly, the length of hospital stay was longer in patients with IOS than those without IOS (median days, 29.0 [IQR, 13.8−58.0] vs. 12.0 [IQR, 7.0−24.0]; p < 0.001). Patients with IOS had higher mortality before ICU discharge (26.1% vs. 7.8%; p < 0.001), before hospital discharge (31.7% vs. 9.4%; p < 0.001), and during the first 90 days after hospital discharge (43.1% vs. 14.0%; p < 0.001), compared with those without IOS (Fig. 1). In the multivariable logistic regression analysis, patients with IOS had higher mortality before ICU discharge (AOR, 1.78; 95% CI, 1.23−2.57; p = 0.002), before hospital discharge (AOR, 1.87; 95% CI, 1.32−2.63; p < 0.001), and at 90 days after hospital discharge (AOR, 2.20; 95% CI, 1.58–3.05; p < 0.001) than patients without IOS, after adjustments for APACHE II score and comorbidities. The Cox proportional hazard model showed that patients with IOS had a hazard ratio of 1.31 in terms of mortality at 90 days after hospital admission (95% CI, 1.04–1.64; p = 0.002) after adjusting for APACHE II score and comorbidities (Fig. 2).
Mortality and length of stay according to the occurrence of IOS. a Patients with stroke had significantly higher mortality at all three time points compared with those without IOS (p < 0.001 in all time points). Patients with hemorrhagic stroke had higher mortality than those with ischemic IOS (p < 0.001 in all time points). b The length of stay in both the ICU and hospital were longer in patients with stroke compared to those without IOS (p < 0.001 in all time points). The length of stay in the ICU and hospital were not significantly different between patients with ischemic IOS and patients with hemorrhagic IOS. All values are presented as median. Error bars indicate interquartile ranges. ICU intensive care unit, IOS ICU-onset stroke
Discussion
This is one of the largest studies reported to date on the rate of newly developed stroke during ICU care in patients with non-neurological disease. We found the incidence of IOS among adult patients admitted to ICU with non-neurological critical illnesses was 1.2% (218/18,604). The proportions of patients with ischemic and hemorrhagic IOS were 83% and 17%, respectively. Cardiovascular surgery was associated with ischemic IOS, and prothrombin time prolongation was associated with hemorrhagic IOS; higher APACHE II scores, mechanical ventilator and ECMO were associated with both ischemic and hemorrhagic IOS. Patients with IOS had high mortality rates before hospital discharge (32%) and at 90 days after hospital discharge (43%), which were approximately twice as high as the mortality rates of patients without IOS. Furthermore, the occurrence of IOS was independently associated with 2.2-fold increased risk of mortality at 90 days from hospital discharge.
The incidence of IOS was higher than expected. During a median 4.0 days of their ICU admission, 1.2 of 100 patients developed IOS, which was much more common compared with general population in Korea (stroke incidence, 232 per 100,000 person-years) [14]. Moreover, risk factors for IOS in the current study were very different from well-known risk factors for community-onset stroke: critical conditions (e.g., APACHE II score, cardiovascular surgery, mechanical ventilation, and ECMO), but not the premorbid conditions (e.g., old age, hypertension, diabetes mellitus, and atrial fibrillation), were related to IOS. Cardiovascular surgery was also a risk factor for in-hospital stroke [4]. Prothrombin time prolongation, which suggests increased bleeding tendency, was associated with hemorrhagic IOS. This is in line with the most common etiology of ICH; coagulopathy from sepsis, liver failure, and hematologic malignancy. We also found that the application of mechanical ventilation and ECMO was related to both ischemic and hemorrhagic IOS. Positive pressure ventilation may provoke thromboembolism by inducing hypercoagulable state, opening unrecognized patent foramen ovale, and new-onset atrial fibrillation [15,16,17,18,19]. In addition, weaning from mechanical ventilation may induce hemodynamic changes and cardiac failure [20]. Exposure of blood to the ECMO circuit may result in the formation and embolization of thrombi. ECMO may also lead to platelet dysfunction and coagulopathy, and the use of anticoagulants may contribute to the occurrence of ICH [21]. These conditions might invoke or trigger both ischemic and hemorrhagic IOSs in vulnerable patients who were admitted to the ICU.
The current study showed that neurological manifestations may differ between ischemic and hemorrhagic IOS. Aphasia and hemiparesis were more common in patients with ischemic IOS than in patients with hemorrhagic IOS, while altered mental status and pupillary changes were more common in patients with hemorrhagic IOS than in patients with ischemic IOS. The diagnosis of stroke based on clinical findings is important in IOS, because there are high risks in transporting patients to the outside of the ICU for neuroimaging studies [22, 23].
It is important to recognize altered mental status as a potential clinical presentation of IOS. The recognition of stroke is probably the first step to perform urgent brain and vascular imaging and allow rapid treatments. In patients admitted to the ICU, however, altered mental status related to sedative agents are likely difficult to be differentiated from altered mental status as the presenting symptom of IOS. The most common reason for delays in stroke recognition in our patients was the use of sedative agents following surgery and mechanical ventilation. Unfortunately, altered mental status was also the most common neurological manifestation of IOS. Thus, delays in symptom recognition were substantial in our patients with altered mental status. To reduce delays in the stroke recognition for patients requiring sedative agents, targeting light sedation, interrupting sedative agents daily, and administering sedative agents with short context-sensitive half-time may be helpful [24, 25]. Patients with hemiparesis and pupillary changes had significantly shorter time intervals from the recognition of stroke symptoms to the performance of neuroimaging studies. Such differences of time intervals according to neurological symptoms may be in part due to the physician’s confidence of the occurrence of IOS, Otherwise, coexisting medical conditions such as unstable vital signs and applications of medical equipment could have interfered with the performance of neuroimaging studies.
Time delays in diagnosing stroke may contribute to low rates of reperfusion therapy, which may result in worse outcomes. Reperfusion therapy was performed in only 7.1% in patients with ischemic IOS (intravenous thrombolysis, 2.7%; intraarterial thrombectomy, 4.4%), which is much lower than the reperfusion therapy rate in our previous study of emergency room treatment of community-onset stroke (intravenous thrombolysis and/or intraarterial thrombectomy, 15.8%; intravenous thrombolysis, 11.4%; intraarterial thrombectomy, 6.4%) as well as that in Korean nation-wide statistics for patients with community-onset stroke (intravenous thrombolysis, 10.7%; and intraarterial thrombectomy, 3.6%) [14, 26]. Notably, in 87% (159/182) of patients with ischemic IOS, the reason to not conduct intravenous thrombolysis was delays in identifying stroke beyond 4.5 h from the last-known-normal time. These patients could have received thrombolytic therapy if their strokes were detected earlier, and such therapy could have improved their outcomes. These findings suggest that special attention is necessary to expedite assessments and therapies for patients with IOS.
The mortality rate at any stage from ICU discharge to 90 days after discharge was significantly higher in patients with IOS than those without IOS. We evaluated increased risk of mortality by stroke both at 90 days after hospital discharge and 90 days after admission, because stroke onset time was wide-ranging. The mortality rate at 90 days after hospital discharge in patients with IOS was 43%, which is approximately three times higher compared with mortality rate in patients without IOS (14%) and mortality rate in patients with community-onset stroke (13%) [14]. It is uncertain whether the high mortality rate of patients with IOS resulted from IOS per se or if the high rate resulted from an underlying medical illness or an interaction between an underlying illness and IOS. However, the occurrence of IOS was associated with mortality, even after adjustments for APACHE II scores and comorbid conditions. These findings underline the importance of early detection and proper management of stroke in patients admitted to the ICU with non-neurological critical illness. As most patients with IOS are taken care of by general physicians and intensivists who do not specialized in stroke management, the activation of stroke code and specialized teams may be needed for patients with stroke symptoms [27].
Our study has limitations. First, this is a single-center retrospective study; thus, our findings should be interpreted cautiously for patients in other centers. Second, the incidence of IOS might be underestimated in our study. We defined stroke according to the findings on CT and/or MRI images of the brain, but only 22% of ICU patients underwent such neuroimaging studies, so patients who developed stroke but did not undergo neuroimaging studies, due to very unstable vital signs and early death soon after admission to the ICU, may not have been identified as having IOS. Moreover, among patients who underwent brain scans for this study, as many as 20.3% underwent CT scans without MRI. It is possible that CT scans could have missed acute infarcts, because the sensitivity of CT for detecting acute infarct is lower than MRI (diffusion-weighted imaging). Third, neurological outcomes as evaluated with validated scales, such as the modified Rankin Scale, were not available for this retrospective study. The mortality and length of stay at ICU and at hospital may be insufficient to evaluate clinical outcomes of stroke victims.
Conclusions
Patients with initially severe illness, cardiovascular surgery, prothrombin time prolongation, and application of mechanical ventilation and ECMO had high risks for developing acute stroke during their admission to the ICU. Substantial time delays ensued in the evaluation and management of IOS. IOS was associated with increased morbidity and mortality. These results call for strategies for prevention, early detection, and proper managements for IOS.
Data availability
All deidentified data that support the findings of this study are available upon reasonable request to the corresponding author from other researchers if ethical approval is granted.
References
Park HJ, Cho HJ, Kim YD, Lee DW, Choi HY, Kim SM, Heo JH (2009) Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol 16(5):582–588. https://doi.org/10.1111/j.1468-1331.2009.02538.x
Kimura K, Minematsu K, Yamaguchi T (2006) Characteristics of in-hospital onset ischemic stroke. Eur Neurol 55(3):155–159. https://doi.org/10.1159/000093574
Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A, Investigators PCNASRMP (2008) In-hospital stroke in a statewide stroke registry. Cerebrovasc Dis 25(1–2):12–20. https://doi.org/10.1159/000111494
Saltman AP, Silver FL, Fang J, Stamplecoski M, Kapral MK (2015) Care and outcomes of patients with in-hospital stroke. JAMA Neurol 72(7):749–755. https://doi.org/10.1001/jamaneurol.2015.0284
Pilato F, Profice P, Dileone M, Ranieri F, Capone F, Minicuci G, Tagliente D, Florio L, Di Iorio R, Plantone D, Tonali PA, Di Lazzaro V (2009) Stroke in critically ill patients. Minerva Anestesiol 75(5):245–250
Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Lingsma H, van der Lugt A, van Zwam WH, van Oostenbrugge RJ, Roos YB, Majoie CB, Dippel DW, Investigators MRCLEAN (2014) MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials 15:343. https://doi.org/10.1186/1745-6215-15-343
Blacker DJ (2003) In-hospital stroke. Lancet Neurol 2(12):741–746. https://doi.org/10.1016/s1474-4422(03)00586-6
Jeon SB, Koh Y, Choi HA, Lee K (2014) Critical care for patients with massive ischemic stroke. J Stroke 16(3):146–160. https://doi.org/10.5853/jos.2014.16.3.146
Lee H, Lim CW, Hong HP, Ju JW, Jeon YT, Hwang JW, Park HP (2015) Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care 43(2):175–186. https://doi.org/10.1177/0310057x1504300206
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43(3):304–377. https://doi.org/10.1007/s00134-017-4683-6
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1):35–41. https://doi.org/10.1161/01.str.24.1.35
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32(4):891–897. https://doi.org/10.1161/01.str.32.4.891
Kang DW, Han MK, Kim HJ, Yun SC, Jeon SB, Bae HJ, Kwon SU, Kim JS (2012) New ischemic lesions coexisting with acute intracerebral hemorrhage. Neurology 79(9):848–855. https://doi.org/10.1212/WNL.0b013e3182648a79
Kim JY, Kang K, Kang J, Koo J, Kim DH, Kim BJ, Kim WJ, Kim EG, Kim JG, Kim JM, Kim JT, Kim C, Nah HW, Park KY, Park MS, Park JM, Park JH, Park TH, Park HK, Seo WK, Seo JH, Song TJ, Ahn SH, Oh MS, Oh HG, Yu S, Lee KJ, Lee KB, Lee K, Lee SH, Lee SJ, Jang MU, Chung JW, Cho YJ, Choi KH, Choi JC, Hong KS, Hwang YH, Kim SE, Lee JS, Choi J, Kim MS, Kim YJ, Seok J, Jang S, Han S, Han HW, Hong JH, Yun H, Lee J, Bae HJ (2019) Executive summary of stroke statistics in Korea 2018: a report from the epidemiology research council of the Korean stroke society. J Stroke 21(1):42–59. https://doi.org/10.5853/jos.2018.03125
Haitsma JJ, Schultz MJ, Hofstra JJ, Kuiper JW, Juco J, Vaschetto R, Levi M, Zhang H, Slutsky AS (2008) Ventilator-induced coagulopathy in experimental Streptococcus pneumoniae pneumonia. Eur Respir J 32:1599–1606. https://doi.org/10.1183/09031936.00045908
Pragliola C, Di Michele S, Galzerano D (2017) A case of shunting postoperative patent foramen ovale under mechanical ventilation controlled by different ventilator settings. Clin Pract 7:969. https://doi.org/10.4081/cp.2017.969
Seok HY, Seo WK, Eun MY, Kwon DY, Park MH, Oh K (2010) Transient increase in intrathoracic pressure as a contributing factor to cardioembolic stroke. J Clin Neurol 6:212–215. https://doi.org/10.3988/jcn.2010.6.4.212
Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, Benjamin EJ (2013) Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J 165:949–955.e943. https://doi.org/10.1016/j.ahj.2013.03.020
Zochios VA, Keeshan A (2013) Pulmonary embolism in the mechanically-ventilated critically ill patient: is it different? J Intensive Care Soc 14:36–44. https://doi.org/10.1177/175114371301400109
Dres M, Teboul JL, Monnet X (2014) Weaning the cardiac patient from mechanical ventilation. Curr Opin Crit Care 20:493–498. https://doi.org/10.1097/MCC.0000000000000131
Kim HJ, Jeong S, Jeon SB (2017) Documenting the invisible in stroke-like symptoms during extracorporeal membrane oxygenation. Intensive Care Med 43(4):566–567. https://doi.org/10.1007/s00134-016-4659-y
Szem JW, Hydo LJ, Fischer E, Kapur S, Klemperer J, Barie PS (1995) High-risk intrahospital transport of critically ill patients: safety and outcome of the necessary “road trip”. Crit Care Med 23(10):1660–1666. https://doi.org/10.1097/00003246-199510000-00009
Fanara B, Manzon C, Barbot O, Desmettre T, Capellier G (2010) Recommendations for the intra-hospital transport of critically ill patients. Crit Care 14(3):R87. https://doi.org/10.1186/cc9018
Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani (2018) Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 46:e825–e873. https://doi.org/10.1097/CCM.0000000000003299
Kim YS, Lee HJ, Jeon SB (2015) Management of pain and agitation for patients in the intensive care unit. J Neurocrit Care 8(2):53–65. https://doi.org/10.18700/jnc.2015.8.2.53
Jeon SB, Ryoo SM, Lee DH, Kwon SU, Jang S, Lee EJ, Lee SH, Han JH, Yoon MJ, Jeong S, Cho YU, Jo S, Lim SB, Kim JG, Lee HB, Jung SC, Park KW, Lee MH, Kang DW, Suh DC, Kim JS (2017) Multidisciplinary approach to decrease in-hospital delay for stroke thrombolysis. J Stroke 19(2):196–204. https://doi.org/10.5853/jos.2016.01802
Jeon SB, Lee HB, Koo YS, Lee H, Lee JH, Park B, Choi SH, Jeong S, Chang JY, Hong SB, Lim CM, Lee SA (2020) Neurological emergencies in patients hospitalized with non-neurological illness. J Patient Saf. https://doi.org/10.1097/PTS.0000000000000682
Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, Ringleb AP, Lorenzano S, Manelfe C, Bozzao L (1999) Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 30:2280–2284. https://doi.org/10.1161/01.str.30.11.2280
Funding
This study was supported by grants of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant numbers: HI18C1487 and HI18C2383).
Author information
Authors and Affiliations
Contributions
SJ, JYC, and S-BJ contributed to the concept and design of the study. SJ, JYC, SJ, SJ, S-BJ contributed to the acquisition and analysis of the data. SJ and S-BJ contributed to drafting the text, which was reviewed and revised by all co-authors.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethical standards
This study was approved by the institutional review board of Asan Medical Center.
Ethics approval and consent to participate
This study was approved by the institutional review board of Asan Medical Center, and the need for written informed consent was waived because of the retrospective design of the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jo, S., Chang, J.Y., Jeong, S. et al. Newly developed stroke in patients admitted to non-neurological intensive care units. J Neurol 267, 2961–2970 (2020). https://doi.org/10.1007/s00415-020-09955-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09955-5