Abstract
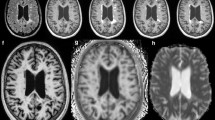
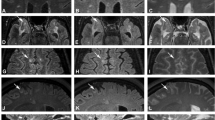
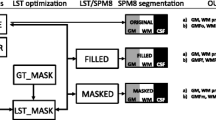
Multiple sclerosis (MS) diagnostic criteria are based upon clinical presentation and presence of white matter hyperintensities on two-dimensional magnetic resonance imaging (MRI) views. Such criteria, however, are prone to false-positive interpretations due to the presence of similar MRI findings in non-specific white matter disease (NSWMD) states such as migraine and microvascular disease. The coexistence of age-related changes has also been recognized in MS patients, and this comorbidity further poses a diagnostic challenge. In this study, we investigated the physiologic profiles within and around MS and NSWMD lesions and their ability to distinguish the two disease states. MS and NSWMD lesions were identified using three-dimensional (3D) T2-FLAIR images and segmented using geodesic active contouring. A dual-echo functional MRI sequence permitted near-simultaneous measurement of blood-oxygen-level-dependent signal (BOLD) and cerebral blood flow (CBF). BOLD and CBF were calculated within lesions and in 3D concentric layers surrounding each lesion. BOLD slope, an indicator of lesion metabolic capacity, was calculated as the change in BOLD from a lesion through its surrounding perimeters. We observed sequential BOLD signal reductions from the lesion towards the perimeters for MS, while no such decreases were observed for NSWMD lesions. BOLD slope was significantly lower in MS compared to NSWM lesions, suggesting decreased metabolic activity in MS lesions. Furthermore, BOLD signal within and around lesions significantly distinguished MS and NSWMD lesions. These results suggest that this technique shows promise for clinical utility in distinguishing NSWMD or MS disease states and identifying NSWMD lesions occurring in MS patients.




Similar content being viewed by others
References
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Liu S, Kullnat J, Bourdette D et al (2013) Prevalence of brain magnetic resonance imaging meeting Barkhof and McDonald criteria for dissemination in space among headache patients. Mult Scler J 19(8):1101–1105. https://doi.org/10.1177/1352458512471874
Seneviratne U, Chong W, Billimoria PH (2013) Brain white matter hyperintensities in migraine: clinical and radiological correlates. Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2012.10.033
Absinta M, Rocca MA, Colombo B et al (2012) Patients with migraine do not have MRI-visible cortical lesions. J Neurol. https://doi.org/10.1007/s00415-012-6571-x
Akman-Demir G, Mutlu M, Kiyat-Atamer A et al (2015) Behçet’s disease patients with multiple sclerosis-like features: discriminative value of Barkhof criteria. Clin Exp Rheumatol 33:S80–S84
Kim SS, Richman DP, Johnson WO et al (2014) Limited utility of current MRI criteria for distinguishing multiple sclerosis from common mimickers: primary and secondary CNS vasculitis, lupus and Sjogren’s syndrome. Mult Scler. https://doi.org/10.1177/1352458513491329
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria THOMPSON, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Barkhof F, Filippi M, Miller DH et al (1997) Comparison of MRI criteria at irst presentation to predict conversion to clinically deinite multiple sclerosis. Brain 120(11):2059–2069. https://doi.org/10.1093/brain/120.11.2059
Toledano M, Weinshenker BG, Solomon AJ (2015) A clinical approach to the differential diagnosis of multiple sclerosis. Curr Neurol Neurosci Rep 15(8):57. https://doi.org/10.1007/s11910-015-0576-7
Solomon AJ, Weinshenker BG (2013) Misdiagnosis of multiple sclerosis: frequency, causes, effects, and prevention. CurrNeurol Neurosci Rep. https://doi.org/10.1007/s11910-013-0403-y
Solomon AJ, Bourdette DN, Cross AH et al (2016) The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology 87:1393–1399. https://doi.org/10.1212/WNL.0000000000003152
Geraldes R, Ciccarelli O, Barkhof F et al (2018) The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol 14:199–213. https://doi.org/10.1038/nrneurol.2018.14
Schmidt R, Enzinger C, Ropele S et al (2006) Subcortical vascular cognitive impairment: Similarities and differences with multiple sclerosis. J Neurol Sci. https://doi.org/10.1016/j.jns.2005.06.018
Geraldes R, Esiri MM, Deluca GC, Palace J Age-related small vessel disease: a potential contributor to neurodegeneration in multiple sclerosis. https://doi.org/10.1111/bpa.12460
Giorgio A, De Stefano N (2016) Advanced structural and functional brain MRI in multiple sclerosis. Semin Neurol. https://doi.org/10.1055/s-0036-1579737
Rocca MA, Colombo B, Pratesi A et al (2000) A magnetization transfer imaging study of the brain in patients with migraine. Neurology 54:507–507. https://doi.org/10.1212/WNL.54.2.507
Mistry N, Abdel-Fahim R, Samaraweera A et al (2016) Imaging central veins in brain lesions with 3-T T2∗-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler. https://doi.org/10.1177/1352458515616700
Samaraweera APR, Clarke MA, Whitehead A et al (2017) The central vein sign in multiple sclerosis lesions is present irrespective of the T2* sequence at 3 T. J Neuroimaging 27:114–121. https://doi.org/10.1111/jon.12367
Sati P, Oh J, Todd Constable R et al (2016) The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. https://doi.org/10.1038/nrneurol.2016.166
Sivakolundu DK, Hansen MR, West KL et al (2019) Three-dimensional lesion phenotyping and physiologic characterization inform remyelination ability in multiple sclerosis. J Neuroimaging 00:1–10. https://doi.org/10.1111/jon.12633
Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci 87(24):9868–9872. https://doi.org/10.1073/pnas.87.24.9868
Kim S-G, Ogawa S (2012) Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab 32:1188–1206. https://doi.org/10.1038/jcbfm.2012.23
Polman CH, Reingold SC, Banwell B, et al Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. https://doi.org/10.1002/ana.22366
Sivakolundu DK, West KL, Maruthy GB et al (2019) Reduced arterial compliance along the cerebrovascular tree predicts cognitive slowing in multiple sclerosis: evidence for a neurovascular uncoupling hypothesis. Mult Scler J. https://doi.org/10.1177/1352458519866605
Sivakolundu DK, West KL, Zuppichini M et al (2020) The neurovascular basis of processing speed diferences in humans: a model-systems approach using multiple sclerosis. NeuroImage. https://doi.org/10.1016/j.neuroimage.2020.116812
Altered task-induced cerebral blood flow and oxygen metabolism underlies motor impairment in multiple sclerosis - Kathryn L West, Dinesh K Sivakolundu, Mark D Zuppichini, Monroe P Turner, Jeffrey S Spence, Hanzhang Lu, Darin T Okuda, Bart Rypma. https://journals.sagepub.com/doi/abs/10.1177/0271678X20908356. Accessed 5 May 2020
Hutchison JL, Lu H, Rypma B (2013) Neural mechanisms of age-related slowing: the ΔCBF/ΔCMRO2 ratio mediates age-differences in BOLD signal and human performance. Cereb Cortex 23:2337–2346
Hoge RD, Atkinson J, Gill B et al (1999) Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 863:849–863
Hoge RD, Atkinson J, Gill B et al (1999) Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci 96:9403–9408
Caselles V, Kimmel R, Sapiro G (1997) Geodesic active contours. Kluwer Academic Publishers, Berlin
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. https://doi.org/10.1006/cbmr.1996.0014
Liu TT, Wong EC (2005) A signal processing model for arterial spin labeling functional MRI. NeuroImage 24:207–215. https://doi.org/10.1016/J.NEUROIMAGE.2004.09.047
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116. https://doi.org/10.1002/mrm.25197
Buxton RB, Wong EC, Frank LR (1998) Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med 39:855–864. https://doi.org/10.1002/mrm.1910390602
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. https://doi.org/10.1016/J.NEUROIMAGE.2006.01.021
Merola A, Germuska MA, Warnert EA et al (2017) Mapping the pharmacological modulation of brain oxygen metabolism: the effects of caffeine on absolute CMRO2 measured using dual calibrated fMRI. NeuroImage 155:331–343. https://doi.org/10.1016/J.NEUROIMAGE.2017.03.028
Box GEP, Tidwell PW (2012) Transformation of the independent variables. Technometrics 4(4):531–550. https://doi.org/10.1080/00401706.1962.10490038
Trapp BD, Stys PK (2009) Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. www.thelancet.com/neurology8. https://doi.org/10.1016/S1474-4422(09)70043-2
Dutta R, Mcdonough J, Yin X et al (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59:478–489. https://doi.org/10.1002/ana.20736
Belov P, Jakimovski D, Krawiecki J et al (2018) Lower arterial cross-sectional area of carotid and vertebral arteries and higher frequency of secondary neck vessels are associated with multiple sclerosis. AJNR Am J Neuroradiol 39:123–130. https://doi.org/10.3174/ajnr.A5469
Porter A, Gladstone JP, Dodick DW (2005) Migraine and white matter hyperintensities. Curr Pain Headache Rep 9(4):289. https://doi.org/10.1007/s11916-005-0039-y
Galluzzi S, Lanni C, Pantoni L et al (2008) White matter lesions in the elderly: pathophysiological hypothesis on the efect on brain plasticity and reserve. J Neurol Sci 273(1–2):3–9. https://doi.org/10.1016/j.jns.2008.06.023
Abdelkarim D, Zhao Y, Turner MP et al (2019) A neural-vascular complex of age-related changes in the human brain: anatomy, physiology, and implications for neurocognitive aging. Neurosc Biobehav Rev 107:927–944. https://doi.org/10.1016/j.neubiorev.2019.09.005
Ge Y, Law M, Johnson G et al (2005) Dynamic susceptibility contrast perfusion MR imaging of multiple sclerosis lesions: characterizing hemodynamic impairment and inflammatory activity. Am J Neuroradiol 26:1539–1547
Lucchinetti C, Brück W, Parisi J et al (2000) Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann Neurol 47:707–717. https://doi.org/10.1002/1531-8249(200006)47:6%3c707:AID-ANA3%3e3.0.CO;2-Q
Filippi M, Rocca MA, Ciccarelli O et al (2016) MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 15:292–303. https://doi.org/10.1016/S1474-4422(15)00393-2
Chen JJ, Carletti F, Young V et al (2016) MRI diferential diagnosis of suspected multiple sclerosis. Clin Radiol 71(9):815–827. https://doi.org/10.1016/j.crad.2016.05.010
Filippi M, Rocca MA (2008) Multiple sclerosis and allied white matter diseases. Neurol Sci. https://doi.org/10.1007/s10072-008-1007-1
Acknowledgements
We thank all the patients and volunteers who participated in this research efforts. We thank our lab research interns and coordinators for their help in recruiting patients and volunteering for the study. Special thanks to Jeffery Spence, and Hanzhang Lu for their scientific input regarding the study. Funding: This work was supported by a National Multiple Sclerosis Society Research Grant (RG-1507-04951) to BR and DO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
KW, MZ, AW, XG, TM, AB, YW, TS, and BR report no disclosures. DO received advisory and consulting fees from Celgene, EMD Serono, Genentech, Genzyme, and Novartis and research support from Biogen. DS, BN, and DO have a patent pending related to the submitted work.
Ethics approval
This study was approved by the University of Texas Southwestern Medical Center Institution Review Board. Written informed consent was obtained from all participants prior to study participation.
Rights and permissions
About this article
Cite this article
Sivakolundu, D.K., West, K.L., Zuppichini, M.D. et al. BOLD signal within and around white matter lesions distinguishes multiple sclerosis and non-specific white matter disease: a three-dimensional approach. J Neurol 267, 2888–2896 (2020). https://doi.org/10.1007/s00415-020-09923-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09923-z