Abstract
Neurologists managing women with Multiple Sclerosis (MS) need information about the safety of disease modifying drugs (DMDs) during pregnancy. However, this knowledge is limited. The present study aims to summarize previous studies by performing a systematic review and meta-analyses. The terms “multiple sclerosis” combined with DMDs of interest and a broad profile for pregnancy terms were used to search Embase and Medline databases to identify relevant studies published from January 2000 to July 2019.1260 studies were identified and ten studies met our inclusion criteria. Pooled risk ratios (RR) of pregnancy and birth outcomes in pregnancies exposed to DMDs compared to those not exposed were calculated using a random effects model. For spontaneous abortion RR = 1.14, 95% CI 0.99–1.32, for preterm births RR = 0.93, 95% CI 0.72–1.21 and for major congenital malformations RR = 0.86, 95% CI 0.47–1.56. The most common major congenital malformations reported in MS patients exposed to MS drugs were atrial septal defect (ASD) (N = 4), polydactyly (N = 4) and club foot (N = 3), which are among the most prevalent birth defects observed in the general population. In conclusion, interferons, glatiramer acetate or natalizumab, do not appear to increase the risk for spontaneous abortions, pre-term birth or major congenital malformations. There were very few patients included that were exposed to fingolimod, azathioprine and rituximab; therefore, these results cannot be generalized across drugs. Future studies including internal comparators are needed to enable treating physicians and their patients to decide on the best treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic neurological disease that affects more women than men, with a female-to-male ratio of 2:1 [1]. MS is generally diagnosed during childbearing age [1]. Symptoms associated with MS may occur in isolated attacks or build up over time. Between attacks, symptoms may disappear completely; however, permanent neurological problems often remain. Hence, the initiation of early-treatment with disease modifying drugs (DMDs) is recommended [2].
Neurologists managing women with MS who wish to conceive need to use a benefit-risk approach and provide adequate advice to their patients. Physicians also need to be prepared in case women taking DMDs become pregnant given that many pregnancies occur unplanned, and therefore, women with MS may inadvertently take DMDs whilst pregnant. It is essential to balance the risk of DMDs during pregnancy to the fetus as compared to the risk of inadequate treatment in the mother. During pregnancy lack of treatment may lead to the development of irreversible disability, since stopping an efficacious drug may induce a relapse [3].
Despite previous systematic reviews and studies [4,5,6], knowledge about the consequences of treatment with DMDs during pregnancy is limited. Based on animal studies and as a result of limited data in humans, the Food and Drug Administration (FDA) assigned category C to the use of most of the DMDs during pregnancy. This category states: “Risk not ruled out: Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks”. The use of pregnancy risk categories presented a challenge for many health care providers, since it did not include enough detailed information related to the safety and efficacy of medications in pregnancy and lactation to assist them in making more evidence-based decisions. As a result, in December 2014 FDA introduced the Pregnancy and Lactation Labeling Rule (PLLR) which removed the pregnancy letter categories. In place of these pregnancy categories, the PLLR requires narrative explanations of risk and supporting data [7]. The EMA provides a decision scheme that helps determine whether or not a contraindication during pregnancy should be settled in the labelling [8]. This guideline considers all non-clinical and clinical data to make an integrated approach in the risk assessment. In recent years, the landscape of MS treatment has changed, with an increasing number of treatment options that have a long-lasting disease modifying effect. Due to the longer half-lives, there is potentially a longer impact during pregnancy. Studies in pregnant women with newer DMDs are scarce.
The aim of this meta-analysis is to evaluate pregnancy outcomes in women with MS treated with DMDs. The results will add to the existing evidence for women with MS considering pregnancy and for neurologists deciding on treatment around pregnancy and counselling women with unplanned pregnancies. This study will also help understand what evidence is lacking in order to provide guidance on what to consider when performing future studies that evaluate clerosis MS treatment during pregnancy.
Methods
Search strategy
The PRISMA 2009 guidelines were followed throughout the study [9]. Searches in Embase and Medline were conducted May–August 2019 to identify relevant publications from the period January 2000 to August 2019. The term multiple sclerosis was combined together with DMDs of interest with a broad profile for pregnancy terms. DMDs of interest were: interferon β-1a, interferon β-1b, PEG interferon β-1a, alemtuzumab, cladribine, dimethyl fumarate, fingolimod, glatiramer acetate, laquinimod, natalizumab, teriflunomide, methotrexate, cyclophosphamide, mycophenolate mofetil, azathioprine, rituximab, mitoxantrone teriflunomide, laquinimod, natalizumab, ofatumumab, rituximab, ocrelizumab, disease modifying, DMD, DMDs B-cell therapy. The pregnancy terms used were: pregnancy, gravidity, prenatal, maternal, gestation, pregnancies, pregnant, pregnanc*, fetal, foetal and congenital. The search was restricted to publications in English. The search was performed using the databases’ controlled vocabulary as well as free-text terms including various spellings and synonyms. Duplicates were removed.
Eligibility criteria
Titles and abstracts were screened to identify peer-reviewed studies which measured the effects of MS drugs on pregnancy. Only studies in which the exposure occurred in utero and compared patients exposed to an MS drug against MS patients without treatment or against the general population were included. Studies which mixed unexposed and exposed in the same group were excluded (e.g., MS patients with and without treatment). However, if the studies included patients taking different DMDs and compared them with one control group they were included as a general DMD group. Interventional studies (e.g., randomized clinical trials) as well as non-interventional studies (e.g., cohort, case–control, and registries) were included.
To avoid bias, studies where the pregnancy outcome was known when the patient entered the study (e.g., retrospective cases) were excluded. Therefore, the pregnancy outcomes were assessed independently of the knowledge regarding pregnancy exposure.
Both primary data collection (e.g., registry studies) as well as studies that included secondary sources (e.g., claims databases) of data were considered. Abstract, reviews, case reports, case series and spontaneous reports were excluded. There was no exclusion based on the pregnancy outcomes, all outcomes available were included.
Data extraction
Two reviewers (MT, MS) independently reviewed the search results for inclusion narrowing potential studies successively in three stages: by title, by abstract, and by full manuscript. Disagreements regarding eligibility were discussed amongst all authors. The quality of eligible studies was independently assessed by SLL and CW using the New Castle Ottawa scale (NOS) [9]. Studies with NOS scores greater than six points were considered to be of high quality. Data from all selected articles were extracted by two authors (MT and SLL) and a quality control, by detecting inconsistencies, was performed by another author (CW).
Statistical analysis
The primary analyses consisted of calculating pooled risk ratios (RR) of pregnancy and birth outcomes (spontaneous abortions, pre-term birth and congenital malformations) in pregnancies exposed to multiple sclerosis drugs compared to those not exposed. All MS drugs were studied together overall, and when possible stratified by drug class [interferons, natalizumab and glatiramer acetate (GA)] to study each individual DMD separately. The pooled RR of all meta-analyses were calculated using a random effects model. The relative weight for each study is calculated as the inverse of the sum of the individual study variance plus the between study variance. The weight for each study is then the relative weight expressed as a proportion of all the relative weights of the studies in the analysis. Therefore, the weight for any study will vary according not only to the number of events occurring in that study and the size of the study, but also to the number of events and size in other studies and also how consistent the study effect is between the studies. The data were analyzed using STATA Version 15.
The secondary analyses consisted of calculating the pooled prevalence rate of the pregnancy outcomes which had two or more published studies in untreated as well as treated MS patients. MetaXL software was used to estimate the pooled prevalence which uses a double arcsine transformation [10].
For all outcomes, except congenital malformations, “all pregnancies” (live birth, spontaneous abortion, or termination of pregnancy) were used as the denominator. In relation to major congenital malformations, all studies used “live births”; therefore, live births were used as the denominator. Heterogeneity was assessed using the I2 statistics. Values of 25%, 50% and 75% for I2 represented low, medium and high heterogeneity, respectively [11]. Publication bias was considered using funnel plots and assessed with Harbord's modified test for small-study effects.
Results
Study selection and characteristics
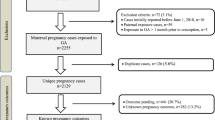
The study search and selection processes are described in detail in Fig. 1. The initial search yielded 1260 publications. After removing duplicates 891 publications were identified. The titles and abstracts were then screened; 832 studies were out of scope and 59 studies were assessed in full. Only ten studies met our inclusion criteria. These ten studies were considered to be of high quality based on the NCO scale (NOS) and were included in the meta-analyses.
Studies included
Table 1 provides characteristics of the included studies. The studies were from Germany (N = 4), Canada (N = 2), Spain, Italy, US and Worldwide. The studies from Germany came from the same cohort; however, the data were not duplicated as the studies selected included different dates or medications. All of the studies were observational studies.
The drugs that were studied the most were interferon, glatiramer acetate, natalizumab. There were very few patients included that were treated with fingolimod, azathioprine or rituximab, MacDonald et al. [12] did not stratify their analyses by type of drug and only conducted their analyses as overall. Therefore, it was not possible to include their data in the drug-specific analyses. Five of the studies only included patients exposed to DMD during the first trimester [5, 13,14,15,16]. None of the studies stratified by trimester of exposure. All of the studies, except one, included as control patients with multiple sclerosis that were not exposed to disease modifying drugs. The comparison group in the study by Ebrahimi et al. [16] included a mixture of multiple sclerosis patients treated with other DMDs than natalizumab and untreated MS patients. Therefore, the data from this study were only included in the pooled estimated prevalence but not in the meta-analysis of risk ratios.
The birth outcomes in which there were enough studies to conduct a meta-analysis were spontaneous abortions, pre-term births and congenital malformations. All studies defined spontaneous abortions as fetal loss before week 22nd of pregnancy. Pre-term birth was defined in all studies as birth before week 37th of pregnancy. All of the studies did not give a definition for adjudication of the malformations nor stated what system they used to define them, they just referred to them as “major malformations”, “major structural malformations” or “major birth defects”. There was no standardization in the definition or wording used. All of the studies, except one, specified that they were major malformations. The study that only referred to them as “congenital malformations” reported that there were none present [13].
Meta-analysis of risk ratios
Figures 2, 3 and 4 present meta-analysis for spontaneous abortions (from eight studies), pre-term births (from seven studies) and major congenital malformations (from eight studies) both overall for all MS drugs as well as stratified by drug class. The RR for spontaneous abortion was RR = 1.14, 95% CI 0.99–1.320, for preterm births RR = 0.93, 95% CI 0.72–1.21 and for major congenital malformations RR = 0.86, 95% CI 0.47–1.56 when compared to MS without treatment. The evidence for heterogeneity was low for spontaneous abortions (I2 = 0%) and major congenital malformations (I2 = 26.1) and medium for preterm birth (I2 = 46.0%). There was no evidence of publication bias.
Pooled prevalence
Table 2 presents the pooled estimated prevalence for spontaneous abortions, premature births and major congenital malformations in untreated as well as treated MS patients. The prevalence for untreated MS patients was: spontaneous abortions 10.9% (95% CI 5.2–18.3) premature birth 12.1% (7.4–17.7) and major congenital malformations 4.2% (2.7–6.1). The prevalence for treated MS patients was: spontaneous abortions 11.6% (7.4–16.7), premature birth 12.12% (9.0–15.6) and major congenital malformations 3.0% (1.8–4.4). The I2 value was extremely high for each pooled outcome, indicating the extremely wide variation in reported prevalences for these outcomes.
Major malformations
The most common major congenital malformations in MS patients exposed to MS drugs were atrial septal defect (ASD) (N = 4), polydactyly (N = 4) and club foot (N = 3). Each case of atrial septal defect was present in patients exposed to different drugs (natalizumab, β-interferon, GA and Mitoxantrone). For the unexposed MS patients most of the studies did not specify what major malformation was present (Table 3). There were only two studies that reported one dysmelia of the tibia and fibula and three ASD [13, 14].
Discussion
The objective of this meta-analysis was to assess if patients treated for MS had an increased risk of adverse pregnancy outcomes compared to MS untreated patients. The outcomes that were possible to study were spontaneous abortions, pre-term live births and major congenital malformations in live births. The results showed that treatment in general did not appear to be associated with these adverse pregnancy outcomes, with the confidence intervals excluding relative risks greater than 50%. Given the limited number of studies there was not the power to evaluate MS drugs separately in an adequate manner. The results were driven by interferon, GA and natalizumab; therefore, it is not possible to generalize to other drugs such as fingolimod, azathioprine or rituximab. Given the diverse mechanisms by which these DMDs work, understanding each DMD individually is highly important; therefore, there is a need for studies with large sample sizes that present their results stratified by type of drug.
The biggest limitation was the number of available published studies and the small sample sizes in these studies. Small sample sizes might be partially due to labels restricting the use of most MS drugs during pregnancy. There was only one study which used a national commercially insurance population which included a large sample size (574 DMD exposed); however, the authors do not present their data stratified by type of drug, and therefore, it was only possible to stratify the results for interferons, natalizumab and GA. There were 13 studies which we had to exclude, because they had not included controls. This shows that there is a great interest in studying the safety of MS drugs during pregnancy; however, there is a need for studies using internal comparators to assess the risk comparing patients taking MS drugs vs patients not taking medication as well as studying in the same study different MS drugs and taking into account confounding factors.
A limitation of these meta-analyses and pregnancy studies in general is the lack of adjustment for confounding factors such as age, comorbidities, comedications, trimester of exposure and environment. Future studies should consider large databases to conduct observational studies. These databases have the advantage of providing large populations that increase the power and reduce the risk of reporting bias. In addition, by increasing the size of the population, it will be possible to evaluate the risk given by each drug in each trimester of exposure and evaluate other outcomes. It is likely that most of the patients were exposed to DMDs during the first trimester of pregnancy. However, of all the studies identified, there was only one study which focused on the exposure of natalizumab during the first trimester [16]. In addition, there is a need to understand the safety risk when exposed later in pregnancy. For example, hematological abnormalities (anemia and thrombocytopenia), were reported in babies exposed to natalizumab in late pregnancy [17].Concerning the evaluation of other outcomes, Houtchens et al. [18] using an administrative claims database from the United States compared the pregnancy prevalence complications in women with and without MS. They reported that women with MS when compared with women without MS had a higher frequency of premature labor, infections, cardiovascular disease, anemia/acquired coagulation disorders and neurological malformations. They did not study if DMDs increased the risk; however, they estimated that only 20% of the MS patients were exposed to a DMD during pregnancy. Future studies using large databases will be adequate to study the safety related to different outcomes.
Seven out of the ten studies, mentioned the type of major congenital malformations that were present in the patients taking MS drugs; however, only two presented the major congenital malformations in the control groups. In the MS patients exposed to DMDs there was no specific pattern seen. The most common major congenital malformations were atrial septal defect (ASD) (N = 4), polydactyly (N = 4) and club foot (N = 3), which are among the most prevalent birth defects observed in the general population [19]. In addition, the only studies that presented the major congenital malformations in controls also listed ASD. The cases of polydactyly were present in β-interferon and natalizumab patients and the club foot in patients exposed to GA and natalizumab. The biggest study did not list the major malformations [12]. We contacted the authors and they stated that given that the study was performed in claims database it was not permitted to publish non-aggregated data.
It is important to note that not all the studies used the same definition for major congenital malformation. Even for the same type of congenital malformation, some authors considered it to be major and others minor. For example, Portaccio et al. [15] classified hip dysplasia as a major congenital malformation, and Thiel et al. [14] classified it as a minor congenital malformation. In addition, Thiel did not mention if dysmelia of the tibia and fibula was considered major or minor and Herbstritt et al. [13] considered it major. Classifications such as the EUROCAT and The Metropolitan Atlanta Congenital Defects Program (MACDP) need to be considered at all times when performing pregnancy studies [19, 20]. In addition, there is a need for standardization, and especially if secondary sources of data are used, algorithms to define pregnancy outcomes need to be carefully validated.
In addition to DMDs being recommended not to be used during pregnancy, for most DMDs, the European Medicine Agency recommends adding contraception measures and time on contraception after the last dose of treatment (e.g., fingolimod 2 months) [21]. Therefore, evidence of pregnancy occurrence and its outcomes in women with MS exposed to these DMDs might take years to gather through traditional pregnancy registries. In this study, results coming from primary collection in MS pregnancy registries were similar to those from secondary use of data. Secondary use of data sources with broad country coverage have the potential to identify a much higher number of exposed cases in a more timely manner and minimizes loss to follow-up of pregnant women and infants.
A recently initiated IMI2 project named ConcePTION, which the authors are part of, will enable the use of such data [22]. This meta-analysis aims to provide guidance on a future study that will assess the safety of MS drugs during pregnancy. In the future study we will tackle some of the limitations observed in the studies we present in this meta-analysis which include the stratification of different DMDs, standardization of definitions and adjustment for confounding factors. In conclusion, interferons, GA or natalizumab, do not appear to increase the risk for spontaneous abortions, pre-term birth or major congenital malformations. There were very few patients included that were exposed with fingolimod, azathioprine and rituximab; therefore, these results cannot be generalized across drugs. Future studies including internal comparators are needed to enable treating physicians and their patients to decide on the best treatment options.
References
Multiple Sclerosis International Federation (2013) Atlas of MS 2013. Mapping Multiple Sclerosis Around the World. https://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf. Accessed Jun 2019
Comi G, Radaelli M, Soelberg Sorensen P (2017) Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 389(10076):1347–1356. https://doi.org/10.1016/S0140-6736(16)32388-1
Coyle PK, Oh J, Magyari M, Oreja-Guevara C, Houtchens M (2019) Management strategies for female patients of reproductive potential with multiple sclerosis: an evidence-based review. Mult Scler Relat Disord 32:54–63. https://doi.org/10.1016/j.msard.2019.04.003
Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J (2018) Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology 90(10):e840–e846. https://doi.org/10.1212/WNL.0000000000005065
Lu E, Wang BW, Guimond C, Synnes A, Sadovnick D, Tremlett H (2012) Disease-modifying drugs for multiple sclerosis in pregnancy: a systematic review. Neurology 79(11):1130–1135. https://doi.org/10.1212/WNL.0b013e3182698c64
Thone J, Thiel S, Gold R, Hellwig K (2017) Treatment of multiple sclerosis during pregnancy—safety considerations. Expert Opin Drug Saf 16(5):523–534. https://doi.org/10.1080/14740338.2017.1311321
Pernia S, DeMaagd G (2016) The new pregnancy and lactation labeling rule. PT 41(11):713–715
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. https://doi.org/10.1002/sim.1186
De Las HV, De Andres C, Tellez N, Tintore M, Group ES (2007) Pregnancy in multiple sclerosis patients treated with immunomodulators prior to or during part of the pregnancy: a descriptive study in the Spanish population. Mult Scler 13(8):981–984. https://doi.org/10.1177/1352458507077896
MacDonald SC, McElrath TF, Hernandez-Diaz S (2019) Use and safety of disease-modifying therapy in pregnant women with multiple sclerosis. Pharmacoepidemiol Drug Saf 28(4):556–560. https://doi.org/10.1002/pds.4735
Herbstritt S, Langer-Gould A, Rockhoff M, Haghikia A, Queisser-Wahrendorf A, Gold R, Hellwig K (2016) Glatiramer acetate during early pregnancy: a prospective cohort study. Mult Scler 22(6):810–816. https://doi.org/10.1177/1352458515623366
Thiel S, Langer-Gould A, Rockhoff M, Haghikia A, Queisser-Wahrendorf A, Gold R, Hellwig K (2016) Interferon-beta exposure during first trimester is safe in women with multiple sclerosis-A prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler 22(6):801–809. https://doi.org/10.1177/1352458516634872
Portaccio E, Annovazzi P, Ghezzi A, Zaffaroni M, Moiola L, Martinelli V, Lanzillo R, Brescia Morra V, Rinaldi F, Gallo P, Tortorella C, Paolicelli D, Pozzilli C, De Giglio L, Cavalla P, Cocco E, Marrosu MG, Patti F, Solaro C, Bellantonio P, Uccelli A, Laroni A, Pasto L, Giannini M, Trojano M, Comi G, Amato MP, Society MSSGotIN (2018) Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: Fetal risks. Neurology 90(10):e823–e831. https://doi.org/10.1212/WNL.0000000000005067
Ebrahimi N, Herbstritt S, Gold R, Amezcua L, Koren G, Hellwig K (2015) Pregnancy and fetal outcomes following natalizumab exposure in pregnancy. A prospective, controlled observational study. Mult Scler 21(2):198–205. https://doi.org/10.1177/1352458514546790
Haghikia A, Langer-Gould A, Rellensmann G (2014) Natalizumab use during the third trimester of pregnancy. Nature 71(7):891–895
Houtchens MK, Edwards NC, Phillips AL (2018) Relapses and disease-modifying drug treatment in pregnancy and live birth in US women with MS. Neurology 91(17):e1570
European Comission (2020) European network of population-based registries for the epidemiological surveillance of congenital anomalies. https://eu-rd-platform.jrc.ec.europa.eu/eurocat. Accessed Jan 2020
CDC (2019) Metropolitan atlanta congenital defects program (MACDP). https://www.cdc.gov/ncbddd/birthdefects/macdp.html. Accessed Dec 2019
EMA (2019) European medical agency. Summary of product characteristics. https://www.ema.europa.eu/en/medicines/. Accessed Dec 2019
IMI-Conception Building an ecosystem for better monitoring and communicating safety of medicines use in pregnancy and breastfeeding: validated and regulatory endorsed workflows for fast, optimised evidence generation. (2019). www.imi-conception.eu/. Accessed Dec 2019
Boskovic R, Wide R, Wolpin J, Bauer DJ, Koren G (2005) The reproductive effects of beta interferon therapy in pregnancy: a longitudinal cohort. Neurology 65(6):807–811. https://doi.org/10.1212/01.wnl.0000180575.77021.c4
Weber-Schoendorfer C, Schaefer C (2009) Multiple sclerosis, immunomodulators, and pregnancy outcome: a prospective observational study. Mult Scler 15(9):1037–1042. https://doi.org/10.1177/1352458509106543
Lu E, Dahlgren L, Sadovnick A, Sayao A, Synnes A, Tremlett H (2012) Perinatal outcomes in women with multiple sclerosis exposed to disease-modifying drugs. Mult Scler 18(4):460–467. https://doi.org/10.1177/1352458511422244
Nguyen AL, Havrdova EK, Horakova D, Izquierdo G, Kalincik T, van der Walt A, Terzi M, Alroughani R, Duquette P, Girard M, Prat A, Boz C, Sola P, Ferraro D, Lugaresi A, Lechner-Scott J, Barnett M, Grand'Maison F, Grammond P, Ramo-Tello C, Turkoglu R, McCombe P, Pucci E, Trojano M, Granella F, Spitaleri D, Van Pesch V, Soysal A, Oreja-Guevara C, Verheul F, Vucic S, Hodgkinson S, Slee M, Ampapa R, Prevost J, Menoyo JLS, Skibina O, Solaro C, Olascoaga J, Shaw C, Madsen KG, Naidoo K, Hyde R, Butzkueven H, Jokubaitis V, Group MSS (2019) Incidence of pregnancy and disease-modifying therapy exposure trends in women with multiple sclerosis: a contemporary cohort study. Mult Scler Relat Disord 28:235–243. https://doi.org/10.1016/j.msard.2019.01.003
Acknowledgements
The publication is part of the activities within the ConcePTION project. It has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant agreement No 821520. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
SLL and YG are an employee of Novartis, MS is employee of Merck, and MT is contracted by Novartis AG.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lopez-Leon, S., Geissbühler, Y., Sabidó, M. et al. A systematic review and meta-analyses of pregnancy and fetal outcomes in women with multiple sclerosis: a contribution from the IMI2 ConcePTION project. J Neurol 267, 2721–2731 (2020). https://doi.org/10.1007/s00415-020-09913-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09913-1