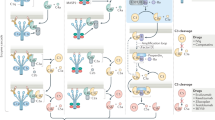
Abstract
The complement system is a powerful member of the innate immune system. It is highly adept at protecting against pathogens, but exists in a delicate balance between its protective functions and overactivity, which can result in autoimmune disease. A cascade of complement proteins that requires sequential activation, and numerous complement regulators, exists to regulate a proportionate response to pathogens. In spite of these mechanisms there is significant evidence for involvement of the complement system in driving the pathogenesis of variety of diseases including neuromyelitis optica spectrum disorders (NMOSD) and myasthenia gravis (MG). As an amplification cascade, there are an abundance of molecular targets that could be utilized for therapeutic intervention. Clinical trials assessing complement pathway inhibition in both these conditions have recently been completed and include the first randomized placebo-controlled trial in NMOSD showing positive results. This review aims to review and update the reader on the complement system and the evolution of complement-based therapeutics in these two disorders.
Similar content being viewed by others
Change history
15 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00415-021-10896-w
17 October 2019
A Correction to this paper has been published: https://doi.org/10.1007/s00415-019-09568-7
References
Brekke OH, Michaelsen TE, Aase A et al (1994) Human IgG isotype-specific amino acid residues affecting complement-mediated cell lysis and phagocytosis. Eur J Immunol 24:2542–2547. https://doi.org/10.1002/eji.1830241042
Petersen JG, Dorrington KJ (1974) An in vitro system for studying the kinetics of interchain disulfide bond formation in immunoglobulin G. J Biol Chem 249:5633–5641
van der Zee JS, van Swieten P, Aalberse RC (1986) Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol 137:3566–3571
Angal S, King DJ, Bodmer MW et al (1993) A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol 30:105–108
Schuurman J, Perdok GJ, Gorter AD, Aalberse RC (2001) The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol Immunol 38:1–8
van der Neut Kolfschoten M, Schuurman J, Losen M et al (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317:1554–1557. https://doi.org/10.1126/science.1144603
Morgan BP, Harris CL (2015) Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov 14:857–877. https://doi.org/10.1038/nrd4657
Brodsky RA (2014) Paroxysmal nocturnal hemoglobinuria. Blood 124:2804–2811. https://doi.org/10.1182/blood-2014-02-522128
Goodfellow RM, Williams AS, Levin JL et al (2000) Soluble complement receptor one (sCR9) inhibits the development and progression of rat collagen-induced arthritis. Clin Exp Immunol 119:210–216. https://doi.org/10.1046/J.1365-2249.2000.01129.X
Oberholzer J, Yu D, Triponez F et al (1999) Decomplementation with cobra venom factor prolongs survival of xenografted islets in a rat to mouse model. Immunology 97:173–180. https://doi.org/10.1046/J.1365-2567.1999.00742.X
Rother RP, Rollins SA, Mojcik CF et al (2007) Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol 25:1256–1264. https://doi.org/10.1038/nbt1344
Pittock SJ, Lennon VA, McKeon A et al (2013) Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. https://doi.org/10.1016/S1474-4422(13)70076-0
Howard JF, Barohn RJ, Cutter GR et al (2013) A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve 48:76–84. https://doi.org/10.1002/mus.23839
Stegall MD, Diwan T, Raghavaiah S et al (2011) Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11:2405–2413. https://doi.org/10.1111/j.1600-6143.2011.03757.x
Levy M, Mealy MA (2014) Purified human C1-esterase inhibitor is safe in acute relapses of neuromyelitis optica. Neurol Neuroimmunol NeuroInflammation 1:1–5. https://doi.org/10.1212/NXI.0000000000000005
Kalbitz M, Fattahi F, Herron TJ et al (2016) Complement destabilizes cardiomyocyte function in vivo after polymicrobial sepsis and in vitro. J Immunol 197:2353–2361. https://doi.org/10.4049/jimmunol.1600091
Grumach AS, Kirschfink M (2014) Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol Immunol 61:110–117
Westra D, Kurvers RAJ, Van den Heuvel LP et al (2014) Compound heterozygous mutations in the C6 gene of a child with recurrent infections. Mol Immunol. https://doi.org/10.1016/j.molimm.2013.11.023
Orren A, Owen EP, Henderson HE et al (2012) Complete deficiency of the sixth complement component (C6Q0), susceptibility to Neisseria meningitidis infections and analysis of the frequencies of C6Q0 gene defects in South Africans. Clin Exp Immunol. https://doi.org/10.1111/j.1365-2249.2011.04525.x
Yih Chen J, Ling WuY, Yin Mok M et al (2016) Effects of complement C4 gene copy number variations, size dichotomy, and C4A deficiency on genetic risk and clinical presentation of systemic lupus erythematosus in East Asian populations. Arthritis Rheumatol 68:1442–1453. https://doi.org/10.1002/art.39589
Fleming SD, Shea-Donohue T, Guthridge JM et al (2002) Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol 169:2126–2133
Dunkelberger JR, Song W-C (2009) Complement in host immunity 34 complement and its role in innate and adaptive immune responses. Cell Res 20:34–5034. https://doi.org/10.1038/cr.2009.139
Baudino L, Sardini A, Ruseva MM et al (2014) C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proc Natl Acad Sci 111:1503–1508. https://doi.org/10.1073/pnas.1316877111
Wagner C, Ochmann C, Schoels M et al (2006) The complement receptor 1, CR1 (CD35), mediates inhibitory signals in human T-lymphocytes. Mol Immunol. https://doi.org/10.1016/j.molimm.2005.04.006
Lennon VA, Wingerchuk DM, Kryzer TJ et al (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet (London, England) 364:2106–2112. https://doi.org/10.1016/S0140-6736(04)17551-X
Lennon VA, Kryzer TJ, Pittock SJ et al (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202:473–477. https://doi.org/10.1084/jem.20050304
Wingerchuk DM, Banwell B, Bennett JL et al (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:177–189. https://doi.org/10.1212/WNL.0000000000001729
Misu T, Fujihara K, Nakashima I et al (2005) Intractable hiccup and nausea with periaqueductal lesions in neuromyelitis optica. Neurology 65:1479–1482. https://doi.org/10.1212/01.wnl.0000183151.19351.82
Apiwattanakul M, Popescu BF, Matiello M et al (2010) Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol 68:757–761. https://doi.org/10.1002/ana.22121
Poppe AY, Lapierre Y, Melançon D et al (2005) Neuromyelitis optica with hypothalamic involvement. Mult Scler 11:617–621. https://doi.org/10.1191/1352458505ms1200cr
Pu S, Long Y, Yang N et al (2015) Syndrome of inappropriate antidiuretic hormone secretion in patients with aquaporin-4 antibody. J Neurol 262:101–107. https://doi.org/10.1007/s00415-014-7537-y
Baba T, Nakashima I, Kanbayashi T et al (2009) Narcolepsy as an initial manifestation of neuromyelitis optica with anti-aquaporin-4 antibody. J Neurol 256:287–288. https://doi.org/10.1007/s00415-009-0139-4
Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG (1999) The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 53:1107–1114
Lucchinetti CF, Guo Y, Popescu BFG et al (2014) The pathology of an autoimmune astrocytopathy: lessons learned from neuromyelitis optica. Brain Pathol 24:83–97. https://doi.org/10.1111/bpa.12099
Pittock SJ, Lucchinetti CF (2016) Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathies: a decade later. Ann N Y Acad Sci 1366:20–39. https://doi.org/10.1111/nyas.12794
Hinson SR, Romero MF, Popescu BFG et al (2012) Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci USA 109:1245–1250. https://doi.org/10.1073/pnas.1109980108
Kitley J, Woodhall M, Leite MI et al (2015) Aquaporin-4 antibody isoform binding specificities do not explain clinical variations in NMO. Neurol Neuroimmunol neuroinflammation 2:e121. https://doi.org/10.1212/NXI.0000000000000121
Mortensen SA, Sander B, Jensen RK et al (2017) Structure and activation of C1, the complex initiating the classical pathway of the complement cascade. Proc Natl Acad Sci 114:986–991. https://doi.org/10.1073/pnas.1616998114
Lehmann-Horn K, Schleich E, Hertzenberg D et al (2011) Anti-CD20 B-cell depletion enhances monocyte reactivity in neuroimmunological disorders. J Neuroinflammation 8:146. https://doi.org/10.1186/1742-2094-8-146
Mader S, Gredler V, Schanda K et al (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 8:184. https://doi.org/10.1186/1742-2094-8-184
Zamvil SS, Slavin AJ (2015) Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol neuroinflammation 2:e62. https://doi.org/10.1212/NXI.0000000000000062
Hinson SR, McKeon A, Fryer JP et al (2009) Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4—expressing cells. Arch Neurol 66:1164–1167. https://doi.org/10.1001/archneurol.2009.188
Phuan PW, Ratelade J, Rossi A et al (2012) Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J Biol Chem. https://doi.org/10.1074/jbc.M112.344325
Nishiyama S, Misu T, Nuriya M et al (2016) Complement-dependent and -independent aquaporin 4-antibody-mediated cytotoxicity in human astrocytes: pathogenetic implications in neuromyelitis optica. Biochem Biophys Reports 7:45–51. https://doi.org/10.1016/j.bbrep.2016.05.012
Ingram G, Loveless S, Howell OW et al (2014) Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol Commun 2:53. https://doi.org/10.1186/2051-5960-2-53
Lucchinetti CF, Mandler RN, Mcgavern D et al (2002) A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 125:1450–1461
Hakobyan S, Luppe S, Evans DR et al (2016) Plasma complement biomarkers distinguish multiple sclerosis and neuromyelitis optica spectrum disorder. Mult Scler J. https://doi.org/10.1177/1352458516669002
Lumsden CE (1971) The immunogenesis of the multiple sclerosis plaque. Brain Res 28:365–390
Compston DAS, Morgan BP, Campbell AK et al (1989) Immunocytochemical localization of the terminal complement complex in multiple sclerosis. Neuropathol Appl Neurobiol 15:307–316. https://doi.org/10.1111/j.1365-2990.1989.tb01231.x
Kuroda H, Fujihara K, Takano R et al (2013) Increase of complement fragment C5a in cerebrospinal fluid during exacerbation of neuromyelitis optica. J Neuroimmunol 254:178–182. https://doi.org/10.1016/j.jneuroim.2012.09.002
Nytrova P, Potlukova E, Kemlink D et al (2014) Complement activation in patients with neuromyelitis optica. J Neuroimmunol 274:185–191. https://doi.org/10.1016/j.jneuroim.2014.07.001
Sádaba MC, Tzartos J, Paíno C et al (2012) Axonal and oligodendrocyte-localized IgM and IgG deposits in MS lesions. J Neuroimmunol 247:86–94. https://doi.org/10.1016/j.jneuroim.2012.03.020
Waters P, Jarius S, Littleton E et al (2008) Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol 65:913–919. https://doi.org/10.1001/archneur.65.7.913
Brink BP, Veerhuis R, Breij ECW et al (2005) The pathology of multiple sclerosis is location-dependent: no significant complement activation is detected in purely cortical lesions. J Neuropathol Exp Neurol 64:147–155
Vamvakas EC, Pineda AA, Weinshenker BG (1995) Meta-analysis of clinical studies of the efficacy of plasma exchange in the treatment of chronic progressive multiple sclerosis. J Clin Apher 10:163–170
Linington C, Morgan BP, Scolding NJ et al (1989) The role of complement in the pathogenesis of experimental allergic encephalomyelitis. Brain 112:895–911
Vriesendorp FJ, Flynn RE, Pappolla MA, Koski CL (1997) Soluble complement receptor 1 (sCR59) is not as effective as cobra venom factor in the treatment of experimental allergic neuritis. Int J Neurosci 92:287–298
Mead RJ, Singhrao SK, Neal JW et al (2002) The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol 168:458–465. https://doi.org/10.4049/JIMMUNOL.168.1.458
Weerth SH, Rus H, Shin ML, Raine CS (2003) Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis. Am J Pathol 163:1069–1080. https://doi.org/10.1016/S0002-9440(10)63466-9
Bradl M, Misu T, Takahashi T et al (2009) Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol 66:630–643. https://doi.org/10.1002/ana.21837
Saadoun S, Waters P, Bell BA et al (2010) Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133:349–361. https://doi.org/10.1093/brain/awp309
Doi H, Matsushita T, Isobe N et al (2009) Hypercomplementemia at relapse in patients with anti-aquaporin-4 antibody. Mult Scler 15:304–310. https://doi.org/10.1177/1352458508099139
Hinson SR, McKeon A, Lennon VA (2010) Neurological autoimmunity targeting aquaporin-4. Neuroscience 168:1009–1018
De Carvalho Jennings Pereira WL, Reiche EMV, Kallaur AP, Kaimen-Maciel DR (2015) Epidemiological, clinical, and immunological characteristics of neuromyelitis optica: a review. J Neurol Sci 355:7–17
Hinson SR, Roemer SF, Lucchinetti CF et al (2008) Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med 205:2473–2481. https://doi.org/10.1084/jem.20081241
Ratelade J, Bennett JL, Verkman AS (2011) Evidence against cellular internalization in vivo of NMO-IgG, aquaporin-4, and excitatory amino acid transporter 2 in neuromyelitis optica. J Biol Chem 286:45156–45164. https://doi.org/10.1074/jbc.M111.297275
Hinson SR, Clift IC, Luo N et al (2017) Autoantibody-induced internalization of CNS AQP4 water channel and EAAT2 glutamate transporter requires astrocytic Fc receptor. Proc Natl Acad Sci 114:5491–5496. https://doi.org/10.1073/pnas.1701960114
Stanley KK, Herz J (1987) Topological mapping of complement component C9 by recombinant DNA techniques suggests a novel mechanism for its insertion into target membranes. EMBO J 6:1951–1957
Whittam D, Wilson M, Hamid S et al (2017) What’s new in neuromyelitis optica? A short review for the clinical neurologist. J Neurol 264:2330–2344. https://doi.org/10.1007/s00415-017-8445-8
Hamid SHM, Whittam D, Mutch K et al (2017) What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol 264:2088–2094. https://doi.org/10.1007/s00415-017-8596-7
Jurynczyk M, Messina S, Woodhall MR et al (2017) Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 140:3128–3138. https://doi.org/10.1093/brain/awx276
Bettelli E, Baeten D, Jäger A et al (2006) Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest 116:2393–2402. https://doi.org/10.1172/JCI28334
Krishnamoorthy G, Lassmann H, Wekerle H, Holz A (2006) Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest 116:2385–2392. https://doi.org/10.1172/JCI28330
Saadoun S, Waters P, Owens GP et al (2014) Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun 2:35. https://doi.org/10.1186/2051-5960-2-35
Jarius S, Wildemann B (2013) Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol 23:661–683. https://doi.org/10.1111/bpa.12084
Yang B, Brown D, Verkman AS (1996) The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem 271:4577–4580
Frigeri A, Gropper MA, Umenishi F et al (1995) Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci 108(Pt 9):2993–3002
Folkesson HG, Matthay MA, Frigeri A, Verkman AS (1996) Transepithelial water permeability in microperfused distal airways. Evidence for channel-mediated water transport. J Clin Invest 97:664–671. https://doi.org/10.1172/JCI118463
Sabolić I, Herak-Kramberger CM, Breton S, Brown D (1999) Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10:913–922
Minami Y, Shimada S, Miyahara H et al (1998) Selective expression of mercurial-insensitive water channel (AQP-4) gene in Hensen and Claudius cells in the rat cochlea. Acta Otolaryngol Suppl 533:19–21
Li J, Patil RV, Verkman AS (2002) Mildly abnormal retinal function in transgenic mice without Müller cell aquaporin-4 water channels. Invest Ophthalmol Vis Sci 43:573–579
Jäger K, Reh D, Gebhardt M et al (2010) Expression profile of aquaporins in human nasolacrimal duct epithelium. Curr Eye Res 35:267–273. https://doi.org/10.3109/02713680903572525
Saadoun S, Papadopoulos MC (2015) Role of membrane complement regulators in neuromyelitis optica. Mult Scler 21:1644–1654. https://doi.org/10.1177/1352458515571446
Kitley J, Leite MI, Nakashima I et al (2012) Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 135:1834–1849. https://doi.org/10.1093/brain/aws109
Kitley J, Palace J (2016) Therapeutic options in neuromyelitis optica spectrum disorders. Expert Rev Neurother. https://doi.org/10.1586/14737175.2016.1150178
Elsone L, Kitley J, Luppe S et al (2014) Long-term efficacy, tolerability and retention rate of azathioprine in 103 aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder patients: a multicentre retrospective observational study from the UK. Mult Scler 20:1533–1540. https://doi.org/10.1177/1352458514525870
Montcuquet A, Collongues N, Papeix C et al (2017) Effectiveness of mycophenolate mofetil as first-line therapy in AQP4-IgG, MOG-IgG, and seronegative neuromyelitis optica spectrum disorders. Mult Scler 23:1377–1384. https://doi.org/10.1177/1352458516678474
Gao F, Chai B, Gu C et al (2019) Effectiveness of rituximab in neuromyelitis optica: a meta-analysis. BMC Neurol 19:36. https://doi.org/10.1186/s12883-019-1261-2
Mealy MA, Wingerchuk DM, Palace J et al (2014) Comparison of relapse and treatment failure rates among patients with neuromyelitis optica. JAMA Neurol 71:324. https://doi.org/10.1001/jamaneurol.2013.5699
Gold R, Stangel M, Dalakas MC (2007) Drug Insight: the use of intravenous immunoglobulin in neurology—therapeutic considerations and practical issues. Nat Clin Pract Neurol 3:36–44. https://doi.org/10.1038/ncpneuro0376
Lutz HU, Stammler P, Bianchi V et al (2004) Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood 103:465–472. https://doi.org/10.1182/blood-2003-05
Basta M, Van Goor F, Luccioli S et al (2003) F(ab)’2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med 9:431–438. https://doi.org/10.1038/nm836
Viswanathan S, Wong AHY, Quek AML, Yuki N (2015) Intravenous immunoglobulin may reduce relapse frequency in neuromyelitis optica. J Neuroimmunol 282:92–96. https://doi.org/10.1016/j.jneuroim.2015.03.021
Magraner MJ, Coret F, Casanova B (2013) Estudio del efecto del tratamiento con inmunoglobulinas por vía intravenosa en la neuromielitis óptica. Neurología 28:65–72. https://doi.org/10.1016/j.nrl.2012.03.014
Bichuetti DB, Oliveira EML (2013) Comment on neuromyelitis optica: potential roles for intravenous immunoglobulin. J Clin Immunol 33:307. https://doi.org/10.1007/s10875-012-9808-7
Elsone L, Panicker J, Mutch K et al (2014) Role of intravenous immunoglobulin in the treatment of acute relapses of neuromyelitis optica: experience in 10 patients. Mult Scler J 20:501–504. https://doi.org/10.1177/1352458513495938
Osman C, Jennings R, El-Ghariani K, Pinto A (2019) Plasma exchange in neurological disease. Pract Neurol Practneurol. https://doi.org/10.1136/practneurol-2019-002336
Bonnan M, Cabre P (2012) Plasma exchange in severe attacks of neuromyelitis optica. Mult Scler Int 2012:1–9. https://doi.org/10.1155/2012/787630
Bonnan M, Valentino R, Debeugny S et al (2018) Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry 89:346–351. https://doi.org/10.1136/jnnp-2017-316286
Kleiter I, Gahlen A, Borisow N et al (2016) Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol 79:206–216. https://doi.org/10.1002/ana.24554
Hillmen P, Young NS, Schubert J et al (2006) The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 355:1233–1243. https://doi.org/10.1056/NEJMoa061648
Zuber J, Fakhouri F, Roumenina LT et al (2012) Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 8:643–657. https://doi.org/10.1038/nrneph.2012.214
Kaplan M (2002) Eculizumab (Alexion). Curr Opin Investig Drugs 3:1017–1023
Alexion (2018) Alexion Announces Successful Phase 3 PREVENT Study of Soliris® (Eculizumab) in Patients with Neuromyelitis Optica Spectrum Disorder (NMOSD)| Alexion Pharmaceuticals, Inc. In: Press Release. https://news.alexionpharma.com/press-release/product-news/alexion-announces-successful-phase-3-prevent-study-soliris-eculizumab-pat. Accessed 14 Mar 2019
Pittock SJ, Berthele A, Fujihara K et al (2019) Eculizumab in aquaporin-4—positive neuromyelitis optica spectrum disorder. N Engl J Med. https://doi.org/10.1056/NEJMoa1900866
Tradtrantip L, Duan T, Yeaman MR, Verkman AS (2019) CD55 upregulation in astrocytes by statins as potential therapy for AQP4-IgG seropositive neuromyelitis optica. J Neuroinflammation 16:57. https://doi.org/10.1186/s12974-019-1448-x
Vincent A, Palace J, Hilton-Jones D (2001) Myasthenia gravis. Lancet 357:2122–2128. https://doi.org/10.1016/S0140-6736(00)05186-2
Conti-Fine BM, Milani M, Kaminski HJ (2006) Myasthenia gravis: past, present, and future. J Clin Invest 116:2843–2854. https://doi.org/10.1172/JCI29894
Kusner LL, Losen M, Vincent A et al (2015) Guidelines for pre-clinical assessment of the acetylcholine receptor-specific passive transfer myasthenia gravis model-recommendations for methods and experimental designs. Exp Neurol. https://doi.org/10.1016/j.expneurol.2015.02.025
Patrick J, Lindstrom J (1973) Autoimmune response to acetylcholine receptor. Science 180:871–872
Toyka KV, Drachman DB, Griffin DE et al (1977) Myasthenia Gravis. N Engl J Med 296:125–131. https://doi.org/10.1056/NEJM197701202960301
Newsom-Davis J, Pinching AJ, Vincent A, Wilson SG (1978) Function of circulating antibody to acetylcholine receptor in myasthenia gravis: investigation by plasma exchange. Neurology 28:266–272
Lindstrom JM, Seybold ME, Lennon VA et al (1976) Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 26:1054–1059
Lennon VA, Lambert EH (1980) Myasthenia gravis induced by monoclonal antibodies to acetylcholine receptors. Nature 285:238–240
Rødgaard A, Nielsen FC, Djurup R et al (1987) Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin Exp Immunol 67:82–88
Heinemann S, Bevan S, Kullberg R et al (1977) Modulation of acetylcholine receptor by antibody against the receptor. Proc Natl Acad Sci USA 74:3090–3094
Drachman DB, Angus CW, Adams RN et al (1978) Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med 298:1116–1122. https://doi.org/10.1056/NEJM197805182982004
Loutrari H, Kokla A, Tzartos SJ (1992) Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur J Immunol 22:2449–2452. https://doi.org/10.1002/eji.1830220939
Losen M, Martínez-Martínez P, Phernambucq M et al (2008) Treatment of myasthenia gravis by preventing acetylcholine receptor modulation. Ann N Y Acad Sci 1132:174–179. https://doi.org/10.1196/annals.1405.034
Almon RR, Andrew CG, Appel SH (1974) Serum globulin in myasthenia gravis: inhibition of alpha-bungarotoxin binding to acetylcholine receptors. Science 186:55–57
Gomez CM, Richman DP (1983) Anti-acetylcholine receptor antibodies directed against the alpha-bungarotoxin binding site induce a unique form of experimental myasthenia. Proc Natl Acad Sci USA 80:4089–4093
Hara H, Hayashi K, Ohta K et al (1993) Detection and characterization of blocking-type anti-acetylcholine receptor antibodies in sera from patients with myasthenia gravis. Clin Chem 39:2053–2057
Lang B, Richardson G, Rees J et al (1988) Plasma from myasthenia gravis patients reduces acetylcholine receptor agonist-induced Na+ flux into TE671 cell line. J Neuroimmunol 19:141–148
Gilhus NE (2016) Myasthenia Gravis. N Engl J Med 375:2570–2581. https://doi.org/10.1056/NEJMra1602678
Hoch W, McConville J, Helms S et al (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7:365–368. https://doi.org/10.1038/85520
Vincent A, Huda S, Cao M et al (2018) Serological and experimental studies in different forms of myasthenia gravis. Ann N Y Acad Sci 1413:143–153. https://doi.org/10.1111/nyas.13592
Evoli A, Alboini PE, Damato V et al (2018) Myasthenia gravis with antibodies to MuSK: an update. Ann N Y Acad Sci 1412:82–89. https://doi.org/10.1111/nyas.13518
Park J-Y, Ikeda H, Ikenaga T, Ono F (2012) Acetylcholine receptors enable the transport of rapsyn from the Golgi complex to the plasma membrane. J Neurosci 32:7356–7363. https://doi.org/10.1523/JNEUROSCI.0397-12.2012
Klooster R, Plomp JJ, Huijbers MG et al (2012) Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 135:1081–1101. https://doi.org/10.1093/brain/aws025
Huijbers MG, Zhang W, Klooster R et al (2013) MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc Natl Acad Sci 110:20783–20788. https://doi.org/10.1073/pnas.1313944110
Williams CL, Hay JE, Huiatt TW, Lennon VA (1992) Paraneoplastic IgG striational autoantibodies produced by clonal thymic B cells and in serum of patients with myasthenia gravis and thymoma react with titin. Lab Invest 66:331–336
Skeie GO, Aarli JA, Matre R et al (1997) Titin antibody positive myasthenia gravis patients have a cellular immune response against the main immunogenic region of titin. Eur J Neurol 4:131–137. https://doi.org/10.1111/j.1468-1331.1997.tb00318.x
Mygland ÅSE, Tysnes O-B, Aarli JA et al (1992) Ryanodine receptor autoantibodies in myasthenia gravis patients with a thymoma. Ann Neurol 32:589–591. https://doi.org/10.1002/ana.410320419
Skeie GO, Lunde PK, Sejersted OM et al (1998) Myasthenia gravis sera containing antiryanodine receptor antibodies inhibit binding of [3H]-ryanodine to sarcoplasmic reticulum. Muscle Nerve 21:329–335
Romi F, Skeie GO, Vedeler C et al (2000) Complement activation by titin and ryanodine receptor autoantibodies in myasthenia gravis. A study of IgG subclasses and clinical correlations. J Neuroimmunol 111:169–176
Skeie GO, Aarli JA, Gilhus NE (2006) Titin and ryanodine receptor antibodies in myasthenia gravis. Acta Neurol Scand 113:19–23. https://doi.org/10.1111/j.1600-0404.2006.00608.x
Zisimopoulou P, Evangelakou P, Tzartos J et al (2014) A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 52:139–145. https://doi.org/10.1016/j.jaut.2013.12.004
Zhang B, Shen C, Bealmear B et al (2014) Autoantibodies to agrin in myasthenia gravis patients. PLoS One 9:e91816. https://doi.org/10.1371/journal.pone.0091816
Gallardo E, Martínez-Hernández E, Titulaer MJ et al (2014) Cortactin autoantibodies in myasthenia gravis. Autoimmun Rev 13:1003–1007. https://doi.org/10.1016/j.autrev.2014.08.039
Cortés-Vicente E, Gallardo E, Martínez MÁ et al (2016) Clinical characteristics of patients with double-seronegative myasthenia gravis and antibodies to cortactin. JAMA Neurol 73:1099. https://doi.org/10.1001/jamaneurol.2016.2032
Illa I, Cortés-Vicente E, Martínez MÁ, Gallardo E (2018) Diagnostic utility of cortactin antibodies in myasthenia gravis. Ann N Y Acad Sci 1412:90–94. https://doi.org/10.1111/nyas.13502
Lindstrom JM, Engel AG, Seybold ME et al (1976) Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine recepotr antibodies. J Exp Med 144:739–753
Nastuk WL, Plescia OJ, Osserman KE (1960) Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med 105:177–184
Engel AG, Lambert EH, Gomez MR (1977) A new myasthenic syndrome with end-plate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann Neurol 1:315–330. https://doi.org/10.1002/ana.410010403
Sahashi K, Engel AG, Linstrom JM et al (1978) Ultrastructural localization of immune complexes (IgG and C3) at the end-plate in experimental autoimmune myasthenia gravis. J Neuropathol Exp Neurol 37:212–223
Fazekas A, Komoly S, Bozsik B, Szobor A (1986) Myasthenia gravis: demonstration of membrane attack complex in muscle end-plates. - PubMed - NCBI. Clin Neuropathol 2:78–83
Engel AG, Arahata K (1987) The membrane attack complex of complement at the endplate in myasthenia gravis. Ann N Y Acad Sci 505:326–332
Casali P, Borzini P, Zanussi C (1976) Letter: immune complexes in myasthenia gravis. Lancet (London, England) 2:378
Barohn RJ, Brey RL (1993) Soluble terminal complement components in human myasthenia gravis. Clin Neurol Neurosurg 95:285–290
Kamolvarin N, Hemachudha T, Ongpipattanakul B et al (1991) Plasma C3c in immune-mediated neurological diseases: a preliminary report. Acta Neurol Scand 83:382–387
Basta M, Illa I, Dalakas MC (1996) Increased in vitro uptake of the complement C3b in the serum of patients with Guillain-Barré syndrome, myasthenia gravis and dermatomyositis. J Neuroimmunol 71:227–229
Romi F, Kristoffersen EK, Aarli JA, Gilhus NE (2005) The role of complement in myasthenia gravis: serological evidence of complement consumption in vivo. J Neuroimmunol 158:191–194. https://doi.org/10.1016/j.jneuroim.2004.08.002
Lennon VA, Seybold ME, Lindstrom JM et al (1978) Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J Exp Med 147:973–983
Christadoss P (1988) C5 gene influences the development of murine myasthenia gravis. J Immunol 140:2589–2592
Tüzün E, Scott BG, Goluszko E et al (2003) Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J Immunol 171:3847–3854
Biesecker G, Gomez CM (1989) Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J Immunol 142:2654–2659
Chamberlain-Banoub J, Neal JW, Mizuno M et al (2006) Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin Exp Immunol 146:278–286. https://doi.org/10.1111/j.1365-2249.2006.03198.x
Lin F, Kaminski HJ, Conti-Fine BM et al (2002) Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J Clin Invest 110:1269–1274. https://doi.org/10.1172/JCI16086
Morgan BP, Chamberlain-Banoub J, Neal JW et al (2006) The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol 146:294–302. https://doi.org/10.1111/j.1365-2249.2006.03205.x
Kusner LL, Halperin JA, Kaminski HJ (2013) Cell surface complement regulators moderate experimental myasthenia gravis pathology. Muscle Nerve 47:33–40. https://doi.org/10.1002/mus.23448
Asghar SS, Pasch MC (2000) Therapeutic inhibition of the complement system. Y2K update. Front Biosci 5:E63–E81
Kusner LL, Satija N, Cheng G, Kaminski HJ (2014) Targeting therapy to the neuromuscular junction: proof of concept. Muscle Nerve 49:749–756. https://doi.org/10.1002/mus.24057
Piddlesden SJ, Jiang S, Levin JL et al (1996) Soluble complement receptor 1 (sCR166) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol 71:173–177
Hepburn NJ, Chamberlain-Banoub JL, Williams AS et al (2008) Prevention of experimental autoimmune myasthenia gravis by rat Crry-Ig: a model agent for long-term complement inhibition in vivo. Mol Immunol 45:395–405. https://doi.org/10.1016/j.molimm.2007.06.144
Hepburn NJ, Williams AS, Nunn MA et al (2007) In Vivo Characterization and Therapeutic Efficacy of a C5-specific Inhibitor from the Soft Tick Ornithodoros moubata. J Biol Chem 282:8292–8299. https://doi.org/10.1074/jbc.M609858200
Soltys J, Kusner LL, Young A et al (2009) Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann Neurol 65:67–75. https://doi.org/10.1002/ana.21536
Tüzün E, Li J, Saini SS et al (2007) Pros and cons of treating murine myasthenia gravis with anti-C1q antibody. J Neuroimmunol 182:167–176. https://doi.org/10.1016/j.jneuroim.2006.10.014
Huda R, Tüzün E, Christadoss P (2013) Complement C2 siRNA mediated therapy of myasthenia gravis in mice. J Autoimmun 42:94–104. https://doi.org/10.1016/j.jaut.2013.01.003
Zhou Y, Gong B, Lin F et al (2007) Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J Immunol 179:8562–8567
Evoli A, Tonali PA, Padua L et al (2003) Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 126:2304–2311. https://doi.org/10.1093/brain/awg223
Gajdos P, Chevret S, Clair B et al (1997) Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann Neurol 41:789–796. https://doi.org/10.1002/ana.410410615
Gajdos P, Chevret S, Toyka KV (2012) Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev 12:CD002277. https://doi.org/10.1002/14651858.CD002277.pub4
Dau PC (1982) Plasmapheresis in myasthenia gravis. Prog Clin Biol Res 88:265–285
Kornfeld P, Ambinder EP, Mittag T et al (1981) Plasmapheresis in refractory generalized myasthenia gravis. Arch Neurol 38:478–481. https://doi.org/10.1001/archneur.1981.00510080040003
Goti P, Spinelli A, Marconi G et al (1995) Comparative effects of plasma exchange and pyridostigmine on respiratory muscle strength and breathing pattern in patients with myasthenia gravis. Thorax 50:1080–1086. https://doi.org/10.1136/thx.50.10.1080
Nagayasu T, Yamayoshi T, Matsumoto K et al (2005) Beneficial effects of plasmapheresis before thymectomy on the outcome in myasthenia gravis. Jpn J Thorac Cardiovasc Surg 53:2–7. https://doi.org/10.1007/s11748-005-1001-y
Palace J, Newsom-Davis J, Lecky B (1998) A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology 50:1778–1783
Sanders DB, Hart IK, Mantegazza R et al (2008) An international, phase III, randomized trial of mycophenolate mofetil in myasthenia gravis. Neurology 71:400–406. https://doi.org/10.1212/01.wnl.0000312374.95186.cc
Pasnoor M, He J, Herbelin L et al (2016) A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology 87:57–64. https://doi.org/10.1212/WNL.0000000000002795
Heckmann JM, Rawoot A, Bateman K et al (2011) A single-blinded trial of methotrexate versus azathioprine as steroid-sparing agents in generalized myasthenia gravis. BMC Neurol 11:97. https://doi.org/10.1186/1471-2377-11-97
Heckmann JM, Bateman K (2017) Letter re: a randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology 88:417.2-417. https://doi.org/10.1212/WNL.0000000000003547
Tandan R, Hehir MK, Waheed W, Howard DB (2017) Rituximab treatment of myasthenia gravis: a systematic review. Muscle Nerve 56:185–196. https://doi.org/10.1002/mus.25597
Wolfe GI, Kaminski HJ, Cutter GR (2016) Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 375:2005–2007. https://doi.org/10.1056/NEJMc1611704
Sussman J, Farrugia ME, Maddison P et al (2015) Myasthenia gravis: association of British Neurologists’ management guidelines. Pract Neurol 15:199–206. https://doi.org/10.1136/practneurol-2015-001126
Brodsky RA, Young NS, Antonioli E et al (2008) Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood 111:1840–1847. https://doi.org/10.1182/blood-2007-06-094136
Zuraw BL, Cicardi M, Longhurst HJ et al (2015) Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate. Allergy 70:1319–1328. https://doi.org/10.1111/all.12658
Chen M, Riedl MA (2017) Emerging therapies in hereditary angioedema. Immunol Allergy Clin N Am 37:585–595. https://doi.org/10.1016/j.iac.2017.03.003
Howard JF, Utsugisawa K, Benatar M et al (2017) Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 16:976–986. https://doi.org/10.1016/S1474-4422(17)30369-1
Sheridan D, Yu Z-X, Zhang Y et al (2018) Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS One 13:e0195909. https://doi.org/10.1371/journal.pone.0195909
McKeage K (2019) Ravulizumab: first global approval. Drugs 79:347–352. https://doi.org/10.1007/s40265-019-01068-2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
AJ: Principal Investigator for the ECU301 and ECU302 trials on Eculizumab in neuromyelitis optica in the UK. AJ has also received research grants from Alexion Pharmaceuticals, Biogen Idec and speaker fees from Biogeb, Chugai, Sanofi-Genzyme and Terumo-BCT. MM: Adboard consulting for Alexion Pharmaceuticals. JC, SH, DH and BPM have no conflicts of interest.
Ethical standards statement
This is a review article and does not involve any animal or human research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chamberlain, J.L., Huda, S., Whittam, D.H. et al. Role of complement and potential of complement inhibitors in myasthenia gravis and neuromyelitis optica spectrum disorders: a brief review. J Neurol 268, 1643–1664 (2021). https://doi.org/10.1007/s00415-019-09498-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09498-4