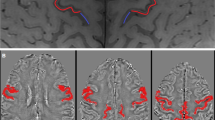
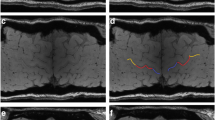
Abstract
There is no reliable objective indicator for upper motor neuron dysfunction in amyotrophic lateral sclerosis (ALS). To determine the clinical significance and potential utility of magnetic resonance (MR) signals, we investigated the relationship between clinical symptoms and susceptibility changes in the motor cortex measured using susceptibility-weighted MR imaging taken by readily available 3-T MRI in clinical practice. Twenty-four ALS patients and 14 control subjects underwent 3-T MR T1-weighted imaging and susceptibility-weighted MR imaging with the principles of echo-shifting with a train of observations (PRESTO) sequence. We analysed relationships between relative susceptibility changes in the motor cortex assessed using voxel-based analysis (VBA) and clinical scores, including upper motor neuron score, ALS functional rating scale revised score, and Medical Research Council sum score on physical examination. Patients with ALS exhibited significantly lower signal intensity in the precentral gyrus on susceptibility-weighted MR imaging compared with controls. Clinical scores were significantly correlated with susceptibility changes. Importantly, the extent of the susceptibility changes in the bilateral precentral gyri was significantly correlated with upper motor neuron scores. The results of our pilot study using VBA indicated that low signal intensity in motor cortex on susceptibility-weighted MR imaging may correspond to clinical symptoms, particularly upper motor neuron dysfunction. Susceptibility-weighted MR imaging may be a useful diagnostic tool as an objective indicator of upper motor neuron dysfunction.





Similar content being viewed by others
References
Chiò A (1999) ISIS Survey: an international study on the diagnostic process and its implications in amyotrophic lateral sclerosis. J Neurol 246:III1–III5
Kasarskis EJ, Tandon L, Lovell MA et al (1995) Aluminum, calcium, and iron in the spinal cord of patients with sporadic amyotrophic lateral sclerosis using laser microprobe mass spectroscopy: a preliminary study. J Neurol Sci 130:203–208
Qian ZM, Wang Q (1998) Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain Res Brain Res Rev 27:257–267
Grosskreutz J, Van Den Bosch L, Keller BU (2010) Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium 47:165–174
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241
Ignjatović A, Stević Z, Lavrnić S et al (2013) Brain iron MRI: a biomarker for amyotrophic lateral sclerosis. J Magn Reson Imaging 38:1472–1479
Yu J, Qi F, Wang N et al (2014) Increased iron level in motor cortex of amyotrophic lateral sclerosis patients: an in vivo MR study. Amyotroph Lateral Scler Frontotemporal Degener 15:357–361
Adachi Y, Sato N, Saito Y et al (2015) Usefulness of SWI for the detection of iron in the motor cortex in amyotrophic lateral sclerosis. J Neuroimaging 25:443–451
Schweitzer AD, Liu T, Gupta A et al (2015) Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclesosis. Am J Roentgenol 204:1086–1092
Chakraborty S, Gupta A, Nguyen T et al (2015) The “motor band sign:” susceptibility-weighted imaging in amyotrophic lateral sclerosis. Can J Neurol Sci 42:260–263
Prell T, Hartung V, Tietz F et al (2015) Susceptibility-weighted imaging provides insight into white matter damage in amyotrophic lateral sclerosis. PLoS One 10:e0131114
Sheelakumari R, Madhusoodanan M, Radhakrishnan A et al (2016) A potential biomarker in amyotrophic lateral sclerosis: can assessment of brain iron deposition with SWI and corticospinal tract degeneration with DTI help? AJNR Am J Neuroradiol 37:252–258
Kakeda S, Yoneda T, Ide S et al (2016) Zebra sign of precentral gyri in amyotrophic lateral sclerosis: a novel finding using phase difference enhanced (PADRE) imaging-initial results. Eur Radiol 26:4173–4183
Oba H, Araki T, Ohtomo K et al (1993) Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 189:843–846
Haacke EM, Xu Y, Cheng YC et al (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618
Haacke EM, Cheng NY, House MJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23:1–25
Liu G, Sobering G, Duyn J et al (1993) A functional MRI technique combining principles of echo-shifting with a train of observations (PRESTO). Magn Reson Med 30:764–768
Tomogane Y, Mori K, Izumoto S et al (2013) Usefulness of PRESTO magnetic resonance imaging for the differentiation of schwannoma and meningioma in the cerebellopontine angle. Neurol Med Chir 53:482–489
Sakurai K, Kawaguchi T, Kawai T et al (2010) Usefulness of 3D-PRESTO imaging in evaluating putaminal abnormality in parkinsonian variant of multiple system atrophy. Neuroradiology 52:809–814
Sakurai K, Imabayashi E, Tokumaru AM et al (2017) Volume of interest analysis of spatially normalized PRESTO imaging to differentiate between parkinson disease and atypical parkinsonian syndrome. Magn Reson Med Sci 16:16–22
Brooks BR, Miller RG, Swash M et al (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Iwata NK, Aoki S, Okabe S et al (2008) Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology 70:528–532
Woo JH, Wang S, Melhem ER et al (2014) Linear associations between clinically assessed upper motor neuron disease and diffusion tensor imaging metrics in amyotrophic lateral sclerosis. PLoS One 9:e105753
Endo H, Sekiguchi K, Ueda T et al (2017) Regional glucose hypometabolic spread within the primary motor cortex is associated with amyotrophic lateral sclerosis disease progression: a fluoro-deoxyglucose positron emission tomography study. eNeurologicalSci 6:74–79
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113
Haller S, Bartsch A, Nguyen D et al (2010) Cerebral microhemorrhage and iron deposition in mild cognitive impairment: susceptibility-weighted MR imaging assessment. Radiology 257:764–773
Gasparotti R, Pinelli L, Liserre R (2011) New MR sequences in daily practice: susceptibility weighted imaging. A pictorial essay. Insights Imaging 2:335–347
Gierga K, Schelhaas HJ, Brunt ER et al (2009) Spinocerebellar ataxia type 6 (SCA6): neurodegeneration goes beyond the known brain predilection sites. Neuropathol Appl Neurobiol 35:515–527
Wang X, Wang H, Xia Y et al (2010) Spinocerebellar ataxia type 6: systematic patho-anatomical study reveals different phylogenetically defined regions of the cerebellum and neural pathways undergo different evolutions of the degenerative process. Neuropathology 30:501–514
Holtkamp M, Zschenderlein R, Brück W et al (2001) Chronic inflammatory demyelinating polyradiculoneuropathy with histologically proven optic neuritis. Acta Neuropathol 101:529–531
Pineda AA, Ogata K, Osoegawa M et al (2007) A distinct subgroup of chronic inflammatory demyelinating polyneuropathy with CNS demyelination and a favorable response to immunotherapy. J Neurol Sci 255:1–6
Cosottini M, Donatelli G, Costagli M et al (2016) High-resolution 7T MR Imaging of the motor cortex in amyotrophic lateral sclerosis. Am J Neuroradiol 37:455–461
Costagli M, Donatelli G, Biagi L et al (2016) Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. Neuroimage Clin 12:965–969
Devine MS, Kiernan MC, Heggie S et al (2014) Study of motor asymmetry in ALS indicates an effect of limb dominance on onset and spread of weakness, and an important role for upper motor neurons. Amyotroph Lateral Scler Frontotemporal Degener 15:481–487
Turner MR, Wicks P, Brownstein CA et al (2011) Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 82:853–854
Devine MS, Pannek K, Coulthard A et al (2015) Exposing asymmetric gray matter vulnerability in amyotrophic lateral sclerosis. Neuroimage Clin 7:782–787
Hadzhieva M, Kirches E, Mawrin C (2014) Review: iron metabolism and the role of iron in neurodegenerative disorders. Neuropathol Appl Neurobiol 40:240–257
Schuster C, Kasper E, Machts J et al (2013) Focal thinning of the motor cortex mirrors clinical features of amyotrophic lateral sclerosis and their phenotypes: a neuroimaging study. J Neurol 260:2856–2864
Bede P, Bokde A, Elamin M et al (2013) Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 84:766–773
Walhout R, Westeneng HJ, Verstraete E et al (2015) Cortical thickness in ALS: towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry 86:288–294
Acknowledgements
The authors thank all of the participants in the present study, members of the Division of Neurology, Kobe University Graduate School of Medicine, and the hospital staff, including Katsusuke Kyotani at the Department of Radiology, Kobe University Hospital. We thank Edanz Group (http://www.edanzediting.com) for editing a draft of this manuscript. We also thank Dr. Takahiro Nakano for confirming the inter-rater reliability of volumes of interest in lateral ventricle cerebrospinal fluid space.
Funding
This work received financial support from the Japan Society for the Promotion of Science KAKENHI Grant number 16K09717. This work was also supported by Development, the Japan Advanced Molecular Imaging Program and Grants-in-Aid for Young Scientists (A) (26713031) and Mochida Memorial Foundation for Medical Pharmaceutical Research to HS.
Author information
Authors and Affiliations
Contributions
HE was involved in the conception and design of the study, acquiring, analysing, and interpreting the data; and writing the manuscript. KS and HS contributed to interpreting the data and substantially revising the manuscript. TU, HK, FK, and TT contributed to revising and approving the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethical standard statement
The medical ethical committee of the Kobe University Graduate School of Medicine allowed this study without sign by the patient. We collected data from medical record which were a part of general medical examination for research purposes. The patient information sufficiently anonymised. In addition, if the patient recognize by any chance, we have been posted the information on the hospital website. On the website, we are providing patients with the opportunity to opt out.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Endo, H., Sekiguchi, K., Shimada, H. et al. Low signal intensity in motor cortex on susceptibility-weighted MR imaging is correlated with clinical signs of amyotrophic lateral sclerosis: a pilot study. J Neurol 265, 552–561 (2018). https://doi.org/10.1007/s00415-017-8728-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8728-0