Abstract
Introduction
Principles of echo shifting with a train of observations (PRESTO) sequence has long echo time which emphasizes the effect of T2* relaxation time and contribute to its high sensitivity to the susceptibility change. The aim of our study was to evaluate the ability of 3D-PRESTO sequence in detecting putaminal hypointensity in patients with parkinsonian variant of multiple system atrophy (MSA-P) and in discriminating between MSA-P and Parkinson's disease (PD).
Methods
The signal intensity of the putamen and localization of abnormality were evaluated on 3D-PRESTO, T2*-weighted (T2*W), and T2-weighted (T2W) sequences in ten patients with MSA-P, 14 with PD, and ten controls. The putaminal signal intensity was assessed in all sequences and graded relative to the palladium. Atrophy of the putamen and posterolateral hyperintensity rim on T2W sequence were also evaluated in MSA-P patients.
Results
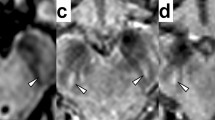
Putaminal hypointensity was more often seen in MSA-P than PD and controls on 3D-PRESTO sequence (p = 0.002) as well as on T2*W sequence (p = 0.003). 3D-PRESTO sequence could reveal lower intensity better than T2*W sequence in four of ten MSA-P cases. Hemi- or bilateral putaminal hypointensity, atrophy, and posterolateral hyperintensity rim were recognized in 90%, 70%, and 70% of ten MSA-P cases, respectively. Three cases revealed hypointensity on 3D-PRESTO sequence without posterolateral hyperintensity rim. Putaminal signal changes occurred in the posterolateral part with a striking lateral to medial gradient in all nine cases with putaminal hypointensity (nine out of nine, 100%).
Conclusions
3D-PRESTO sequence appears to be useful for depicting putaminal hypointensity in MSA-P patients and in differentiating MSA-P from PD.




Similar content being viewed by others
References
Inoue M, Yagishita S, Ryo M et al (1997) The distribution and dynamic density of oligodendroglial cytoplasmic inclusions (GCIs) in multiple system atrophy: a correlation between the density of GCIs and the degree of involvement of striatonigral and olivopontocerebellar systems. Acta Neuropathol 93:585–591
Schwarz J, Weis S, Kraft E et al (1996) Signal changes on MRI and increases in reactive microgliosis, astrogliosis, and iron in the putamen of two patients with multiple system atrophy. J Neurol Neurosurg Psychiatry 60:98–101
Gilman S, Low P, Quinn N et al (1998) Consensus statement on the diagnosis of multiple system atrophy: American Autonomic Society and American Academy of Neurology. Clin Auton Res 8:359–362
Litvan I, Goetz CG, Jankovic J et al (1997) What is the accuracy of the clinical diagnosis of multiple system atrophy? A clinicopathologic study. Arch Neurol 54:937–944
Naka H, Ohshita T, Murata Y et al (2002) Characteristic MRI findings in multiple system atrophy: comparison of the three subtypes. Neuroradiology 44:204–209
Yekhlef F, Ballan G, Macia F et al (2003) Routine MRI for the differential diagnosis of Parkinson's disease, MSA, PSP, and CBD. J Neural Transm 110:151–169
Matsusue E, Fujii S, Kanasaki Y, Sugihara S et al (2008) Putaminal lesion in multiple system atrophy: postmortem MR-pathological correlations. Neuroradiology 50:559–567
Kraft E, Schwarz J, Trenkwalder C et al (1999) The combination of hypointense and hyperintense signal changes on T2-weighted magnetic resonance imaging sequences: a specific marker of multiple system atrophy? Arch Neurol 56:225–228
von Lewinski F, Werner C, Jörn T et al (2007) T2*-weighted MRI in diagnosis of multiple system atrophy. A practical approach for clinicians. J Neurol 254:1184–1188
Kraft E, Trenkwalder C, Auer DP (2002) T2*-weighted MRI differentiates multiple system atrophy from Parkinson's disease. Neurology 59:1265–1267
Liu G, Sobering G, Duvn J et al (1993) A functional MRI technique combining principles of echo-shifting with a train of observations (PRESTO). Magn Reson Med 30:754–768
Gilman S, Low PA, Quinn N et al (1999) Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 163:94–98
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
van Gelderen P, Ramsey NF, Liu G et al (1995) Three-dimensional functional magnetic resonance imaging of human brain on a clinical 1.5-T scanner. Proc Natl Acad Sci U S A 92:6906–6910
Aoki S, Aoki S, Okada Y, Nishimura K et al (1989) Normal deposition of brain iron in childhood and adolescence: MR imaging at 1.5 T. Radiology 172:381–385
Harder SL, Hopp KM, Ward H et al (2008) Mineralization of the deep gray matter with age: a retrospective review with susceptibility-weighted MR imaging. Am J Neuroradiol 29:176–183
Wenning GK, Ben-Shlomo Y, Magalhaes M et al (1995) Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry 58:160–166
Olanow CW (1992) Magnetic resonance imaging in parkinsonism. Neurol Clin 10:405–420
Schrag A, Good CD, Miszkiel K et al (2000) Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 54:697–702
Tsuboyama T, Imaoka I, Shimono T et al (2008) T2*-sensitized high-resolution magnetic resonance venography using 3D-PRESTO technique. Magn Reson Med Sci 7:73–77
Lee HA, Cho HI, Kim SS et al (2004) Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism Relat Disord 10:363–368
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakurai, K., Kawaguchi, T., Kawai, T. et al. Usefulness of 3D-PRESTO imaging in evaluating putaminal abnormality in parkinsonian variant of multiple system atrophy. Neuroradiology 52, 809–814 (2010). https://doi.org/10.1007/s00234-009-0621-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0621-9