Abstract
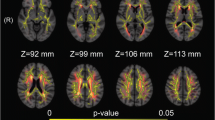
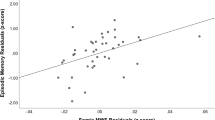
Subjects with metabolic syndrome (MetS) often show worse cognitive performance compared with the healthy population. We investigated whether microstructural white matter abnormalities are associated with cognitive performance in adults with MetS using diffusion tensor MR imaging. A total of 32 subjects with MetS (age 64.8 ± 7.8, 56.25 % female) and 23 age-, gender-, and education-matched healthy controls completed a battery of neuropsychological tests and diffusion tensor imaging (DTI) at 3-T MRI. Brain global and regional volumes, white matter fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (LD) were calculated. The least-square models adjusted for age, sex, HbA1c, hypertension, body mass index, hyperlipidemia, and white matter hyperintensities were used to evaluate the relationship between cognitive function and DTI. The MetS group had worse performance in verbal fluency (VF) and learning and memory function (total VF: T score (p = 0.01), VF: animals T score (p = 0.0001), Hopkins Verbal Learning Test (HVLT): Total recall T score (p = 0.0001), and HVLT: delayed recall T score (p = 0.002), as compared with controls. In the MetS group, abnormalities in diffusivity measures were associated with worse cognitive performance [VF: animals T score and left post-central gyrus-LD (p = 0.0007, r adj 0.4), R angular gyrus-RD (p = 0.0008, r adj 0.3), L supra-marginal gyrus-RD (p = 0.009, r adj 0.2) after adjusting for age, sex, HbA1c, 24 h mean BP, presence of hyperlipidemia, and global white matter hyperintensities]. Microstructural white matter abnormalities in the MetS group might be the underlying mechanisms of worse verbal learning and memory performance.


Similar content being viewed by others
References
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421
Li J, Wang YJ, Zhang M et al (2011) Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology 76:1485–1491. doi:10.1212/WNL.0b013e318217e7a4
Kaffashian S, Dugravot A, Elbaz A et al (2013) Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology 80:1300–1306. doi:10.1212/WNL.0b013e31828ab370
Raffaitin C, Féart C, Le Goff M et al (2011) Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology 76:518–525. doi:10.1212/WNL.0b013e31820b7656
Schmidt R, Launer LJ, Nilsson L-G et al (2004) Magnetic resonance imaging of the brain in diabetes: the Cardiovascular Determinants of Dementia (CASCADE) Study. Diabetes 53:687–692
Kreis R, Ross BD (1992) Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology 184:123–130. doi:10.1148/radiology.184.1.1319074
Manschot SM, Brands AMA, van der Grond J et al (2006) Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 55:1106–1113
Sprandel MCO, Hueb WA, Segre A et al (2015) Alterations in lipid transfers to HDL associated with the presence of coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 14:107. doi:10.1186/s12933-015-0270-8
Reijmer YD, Brundel M, de Bresser J et al (2013) Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care 36:137–144. doi:10.2337/dc12-0493
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Del Bene A, Ciolli L, Borgheresi L et al (2015) Is type 2 diabetes related to leukoaraiosis? an updated review. Acta Neurol Scand 132:147–155. doi:10.1111/ane.12398
Mayer AR, Ling J, Mannell MV et al (2010) A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74:643–650. doi:10.1212/WNL.0b013e3181d0ccdd
Yates KF, Sweat V, Yau PL et al (2012) Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol 32:2060–2067. doi:10.1161/ATVBAHA.112.252759
Segura B, Jurado MA, Freixenet N et al (2009) Microstructural white matter changes in metabolic syndrome: a diffusion tensor imaging study. Neurology 73:438–444. doi:10.1212/WNL.0b013e3181b163cd
Segura B, Jurado MA, Freixenet N et al (2010) White matter fractional anisotropy is related to processing speed in metabolic syndrome patients: a case-control study. BMC Neurol 10:64. doi:10.1186/1471-2377-10-64
Knopman DS, Mosley TH, Catellier DJ et al (2005) Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology 65:876–881. doi:10.1212/01.wnl.0000176074.09733.a8
Tamashiro-Duran JH, Squarzoni P, de Duran FL de S et al (2012) Cardiovascular risk in cognitively preserved elderlies is associated with glucose hypometabolism in the posterior cingulate cortex and precuneus regardless of brain atrophy and apolipoprotein gene variations. AGE 35:777–792. doi:10.1007/s11357-012-9413-y
Franke K, Gaser C, Manor B, Novak V (2013) Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci 5:90. doi:10.3389/fnagi.2013.00090
Srinivasa RN, Rossetti HC, Gupta MK et al (2016) Cardiovascular risk factors associated with smaller brain volumes in regions identified as early predictors of cognitive decline. Radiology 278:198–204. doi:10.1148/radiol.2015142488
Eckel RH, Alberti KGMM, Grundy SM, Zimmet PZ (2010) The metabolic syndrome. Lancet Lond Engl 375:181–183. doi:10.1016/S0140-6736(09)61794-3
Novak V, Last D, Alsop DC et al (2006) Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care 29:1529–1534. doi:10.2337/dc06-0261
Alexander AL, Lee JE, Lazar M, Field AS (2007) Diffusion tensor imaging of the brain. Neurother J Am Soc Exp Neurother 4:316–329. doi:10.1016/j.nurt.2007.05.011
Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM (2005) Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26(8):1215–1227
Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp do PM, Zakszewski E, Field AS (2011) Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect 1(6):423–446. doi:10.1089/brain.2011.0071
Seghier ML (2013) The angular gyrus: multiple functions and multiple subdivisions. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 19:43–61. doi:10.1177/1073858412440596
Hackert VH, den Heijer T, Oudkerk M et al (2002) Hippocampal head size associated with verbal memory performance in nondemented elderly. NeuroImage 17:1365–1372
Chen KHM, Chuah LYM, Sim SKY, Chee MWL (2010) Hippocampal region-specific contributions to memory performance in normal elderly. Brain Cogn 72:400–407. doi:10.1016/j.bandc.2009.11.007
O’Bryant SE, Humphreys JD, Smith GE et al (2008) Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol 65:963–967. doi:10.1001/archneur.65.7.963
Duff K, Schoenberg MR, Scott JG, Adams RL (2005) The relationship between executive functioning and verbal and visual learning and memory. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol 20:111–122. doi:10.1016/j.acn.2004.03.003
Kodl CT, Seaquist ER (2008) Cognitive dysfunction and diabetes mellitus. Endocr Rev 29:494–511. doi:10.1210/er.2007-0034
Cukierman T, Gerstein HC, Williamson JD (2005) Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia 48:2460–2469. doi:10.1007/s00125-005-0023-4
Biessels GJ, Staekenborg S, Brunner E et al (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74. doi:10.1016/S1474-4422(05)70284-2
Hughes TM, Sink KM (2015) Hypertension and its role in cognitive function: current evidence and challenges for the future. Am J Hypertens. doi:10.1093/ajh/hpv180
Hsu J-L, Chen Y-L, Leu J-G et al (2012) Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. NeuroImage 59:1098–1105. doi:10.1016/j.neuroimage.2011.09.041
Zhang J, Wang Y, Wang J et al (2014) White matter integrity disruptions associated with cognitive impairments in type 2 diabetic patients. Diabetes 63:3596–3605. doi:10.2337/db14-0342
Qiu C, Sigurdsson S, Zhang Q et al (2014) Diabetes, markers of brain pathology and cognitive function: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol 75:138–146. doi:10.1002/ana.24063
Yau PL, Kluger A, Borod JC, Convit A (2014) Neural substrates of verbal memory impairments in adults with type 2 diabetes mellitus. J Clin Exp Neuropsychol 36:74–87. doi:10.1080/13803395.2013.869310
Kodl CT, Franc DT, Rao JP et al (2008) Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes 57:3083–3089. doi:10.2337/db08-0724
Kullmann S, Schweizer F, Veit R et al (2015) Compromised white matter integrity in obesity. Obes Rev Off J Int Assoc Study Obes 16:273–281. doi:10.1111/obr.12248
Kullmann S, Callaghan MF, Heni M et al (2015) Specific white matter tissue microstructure changes associated with obesity. NeuroImage 125:36–44. doi:10.1016/j.neuroimage.2015.10.006
Wardlaw JM, Smith C, Dichgans M (2013) Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 12:483–497. doi:10.1016/S1474-4422(13)70060-7
Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9:689–701. doi:10.1016/S1474-4422(10)70104-6
Chung C-C, Pimentel D, Jor’dan AJ et al (2015) Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology 85:450–458. doi:10.1212/WNL.0000000000001820
Last D, Alsop DC, Abduljalil AM et al (2007) Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care 30:1193–1199. doi:10.2337/dc06-2052
Tiehuis AM, Vincken KL, van den Berg E et al (2008) Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia 51:1321–1326. doi:10.1007/s00125-008-1041-9
Arakawa S, Wright PM, Koga M et al (2006) Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke J Cereb Circ 37:1211–1216. doi:10.1161/01.STR.0000217258.63925.6b
Purkayastha S, Fadar O, Mehregan A et al (2014) Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 34:228–234. doi:10.1038/jcbfm.2013.180
Brickman AM, Zahra A, Muraskin J et al (2009) Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res 172:117–120. doi:10.1016/j.pscychresns.2008.11.006
Marstrand JR, Garde E, Rostrup E et al (2002) Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke J Cereb Circ 33:972–976
Markus HS, Lythgoe DJ, Ostegaard L et al (2000) Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J Neurol Neurosurg Psychiatry 69:48–53
Cui X, Abduljalil A, Manor BD et al (2014) Multi-scale glycemic variability: a link to gray matter atrophy and cognitive decline in type 2 diabetes. PLoS One 9:e86284. doi:10.1371/journal.pone.0086284
Reijmer YD, Fotiadis P, Martinez-Ramirez S et al (2015) Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain J Neurol 138:179–188. doi:10.1093/brain/awu316
Saggio ML, Ritter P, Jirsa VK (2016) Analytical operations relate structural and functional connectivity in the brain. PLoS One 11:e0157292. doi:10.1371/journal.pone.0157292
Scaccianoce E, Laganà MM, Baglio F et al (2016) Combined DTI-fMRI analysis for a quantitative assessment of connections between WM bundles and their peripheral cortical fields in verbal fluency. Brain Topogr. doi:10.1007/s10548-016-0516-0
Kesse-Guyot E, Julia C, Andreeva V et al (2015) Evidence of a cumulative effect of cardiometabolic disorders at midlife and subsequent cognitive function. Age Ageing 44:648–654. doi:10.1093/ageing/afv053
Acknowledgments
The study was supported by NIH-NIA 1R01- AG0287601A2, NIH-NIDDK 5R21 DK084463, 1R01DK13902-01A1, American Diabetes Association, Clinical 1-03-CR-23 and 1-06-CR-25 to Dr. Vera Novak. The project described was supported by Grant Number UL1 RR025758-Harvard Clinical and Translational Science Center and M01-RR-01032, from the National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Freddy Alfaro reports no disclosures. Dr. Vasileios-Arsenios Lioutas reports no disclosures. Dr. Daniela Pimentel reports no disclosures. Dr. Chen-Chih Chung reports no disclosures. Dr. Francisco Bedoya reports no disclosures. Dr. Woo-Kyoung Woo Ph.D. reports no disclosures. Dr. Vera Novak M.D. Ph.D. was funded by NIH-NIA 1R01- AG0287601A2, NIH-NIDDK 5R21 DK084463, NIH-NIDDK 1R01DK13902-01A1, American Diabetes Association, Clinical 1-03-CR-23 and 1-06-CR-25.
Ethical standards
All subjects that met inclusion/exclusion criteria signed an Informed consent form (ICF) as approved by the Institutional review board (IRB) at Beth Israel Deaconess Medical Center.
Additional information
F. J. Alfaro and V.-A. Lioutas contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Alfaro, F.J., Lioutas, VA., Pimentel, D.A. et al. Cognitive decline in metabolic syndrome is linked to microstructural white matter abnormalities. J Neurol 263, 2505–2514 (2016). https://doi.org/10.1007/s00415-016-8292-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8292-z