Abstract
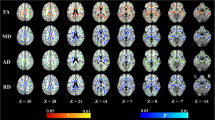
Indexes derived from diffusion tensor imaging (DTI) are sensitive to changes of both T2-hyperintense and normal-appearing brain white matter (WM) in elderly subjects with variable cognitive status. We investigated correlations between global cognitive performance and DTI-derived indexes along the WM tracts in the brain of patients with vascular mild cognitive impairment (MCI) and small vessel disease (SVD). Seventy-six patients with vascular MCI and SVD were assessed through Montreal Cognitive Assessment (MoCA) and Mini Mental State Examination (MMSE) test and underwent DTI examination on a 1.5 T MR scanner. We used Tract Based Spatial Statistics (TBSS) to assess voxel-wise in the entire brain the spatial distribution of the correlation between values of fractional anisotropy, mean, axial/radial diffusivity and global cognitive performance as assessed with MoCA and MMSE tests. All correlations were statistically tested with a significant p-value <0.05 using a family-wise error correction for multiple comparisons. The MoCA score significantly correlated with fractional anisotropy (positive correlation) and mean, axial and radial diffusivity (negative correlations) in WM tracts of cerebral hemispheres and corpus callosum, as well as in the intra-thalamic WM tracts and the superior cerebellar peduncle decussation in the midbrain. No significant correlations were observed for MMSE score. Global cognitive performance, as measured by the MoCA score, in patients with vascular MCI and SVD is associated with microstructural changes in WM tracts underlying intra- and inter-hemispheric cerebral, thalamo-cortical and cerebello-thalamic connections.


Similar content being viewed by others
References
Akhlaghi, H., Yu, J., Corben, L., Georgiou-Karistianis, N., Bradshaw, J. L., Storey, E., et al. (2014). Cognitive deficits in Friedreich ataxia correlate with micro-structural changes in dentatorubral tract. Cerebellum, 13(2), 187–198. https://doi.org/10.1007/s12311-013-0525-4.
Andersson, J. L. R., Jenkinson, M., & Smith, S. M. (2007). Non-linear registration, aka spatial normalisation. FMRIB technical report (pp. TR07JA02).
Arsigny, V., Fillard, P., Pennec, X., & Ayache, N. (2006). Log-Euclidean metrics for fast and simple calculus on diffusion tensors. Magnetic Resonance in Medicine, 56(2), 411–421. https://doi.org/10.1002/mrm.20965.
Balboni, G. C., Bastianini, A., Brizzi, E., Castorina, S., Comparini, L., Donato, R. F., et al. (2000). Anatomia Umana (Vol 2). In Ermes.
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E., & Bousser, M. G. (2009). Cadasil. Lancet Neurology, 8(7), 643–653. https://doi.org/10.1016/S1474-4422(09)70127-9.
Conti, S., Bonazzi, S., Laiacona, M., Masina, M., & Coralli, M. V. (2015). Montreal cognitive assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurological Sciences, 36(2), 209–214. https://doi.org/10.1007/s10072-014-1921-3.
Cook, P. A., Bai, Y., Nedjati-Gilani, S., Seunarine, K. K., Hall, M. G., Parker, G. J., et al. Camino: Open-source diffusion-MRI reconstruction and processing. In 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine, Seattle, WA, USA, 2006 (pp. 2759).
Della Nave, R., Foresti, S., Pratesi, A., Ginestroni, A., Inzitari, M., Salvadori, E., et al. (2007). Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: Correlation with motor and cognitive impairment. AJNR. American Journal of Neuroradiology, 28(7), 1313–1319. https://doi.org/10.3174/ajnr.A0555.
Diciotti, S., Orsolini, S., Salvadori, E., Giorgio, A., Toschi, N., Ciulli, S., Ginestroni, A., Poggesi, A., de Stefano, N., Pantoni, L., Inzitari, D., Mascalchi, M., & VMCI Tuscany investigators. (2017). Resting state fMRI regional homogeneity correlates with cognition measures in subcortical vascular cognitive impairment. Journal of the Neurological Sciences, 373, 1–6. https://doi.org/10.1016/j.jns.2016.12.003.
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR. American Journal of Roentgenology, 149(2), 351–356. https://doi.org/10.2214/ajr.149.2.351.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). "mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198.
Gouw, A. A., Van der Flier, W. M., van Straaten, E. C., Barkhof, F., Ferro, J. M., Baezner, H., et al. (2006). Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: The LADIS study. Journal of Neurology, 253(9), 1189–1196. https://doi.org/10.1007/s00415-006-0193-5.
Goveas, J., O'Dwyer, L., Mascalchi, M., Cosottini, M., Diciotti, S., De Santis, S., et al. (2015). Diffusion-MRI in neurodegenerative disorders. Magnetic Resonance Imaging, 33(7), 853–876. https://doi.org/10.1016/j.mri.2015.04.006.
Hachinski, V., Iadecola, C., Petersen, R. C., Breteler, M. M., Nyenhuis, D. L., Black, S. E., Powers, W. J., DeCarli, C., Merino, J. G., Kalaria, R. N., Vinters, H. V., Holtzman, D. M., Rosenberg, G. A., Wallin, A., Dichgans, M., Marler, J. R., & Leblanc, G. G. (2006). National Institute of Neurological Disorders and Stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke, 37(9), 2220–2241. https://doi.org/10.1161/01.Str.0000237236.88823.47.
Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156.
Jokinen, H., Ryberg, C., Kalska, H., Ylikoski, R., Rostrup, E., Stegmann, M. B., Waldemar, G., Madureira, S., Ferro, J. M., van Straaten, E. C. W., Scheltens, P., Barkhof, F., Fazekas, F., Schmidt, R., Carlucci, G., Pantoni, L., Inzitari, D., Erkinjuntti, T., & on behalf of the LADIS group. (2007). Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: The LADIS study. Journal of Neurology, Neurosurgery, and Psychiatry, 78(5), 491–496. https://doi.org/10.1136/jnnp.2006.096792.
Jokinen, H., Schmidt, R., Ropele, S., Fazekas, F., Gouw, A. A., Barkhof, F., Scheltens, P., Madureira, S., Verdelho, A., Ferro, J. M., Wallin, A., Poggesi, A., Inzitari, D., Pantoni, L., Erkinjuntti, T., & LADIS Study Group. (2013). Diffusion changes predict cognitive and functional outcome: The LADIS study. Annals of Neurology, 73(5), 576–583. https://doi.org/10.1002/ana.23802.
Jones, D. K., Knosche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage, 73, 239–254. https://doi.org/10.1016/j.neuroimage.2012.06.081.
Koski, L. (2013). Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovascular Diseases, 36(1), 6–18. https://doi.org/10.1159/000352051.
Lamar, M., Catani, M., Price, C. C., Heilman, K. M., & Libon, D. J. (2008). The impact of region-specific leukoaraiosis on working memory deficits in dementia. Neuropsychologia, 46(10), 2597–2601. https://doi.org/10.1016/j.neuropsychologia.2008.04.007.
Leemans, A., & Jones, D. K. (2009). The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. https://doi.org/10.1002/mrm.21890.
Mascalchi, M., Ginestroni, A., Toschi, N., Poggesi, A., Cecchi, P., Salvadori, E., Tessa, C., Cosottini, M., de Stefano, N., Pracucci, G., Pantoni, L., Inzitari, D., Diciotti, S., & VMCI Tuscany investigators. (2014). The burden of microstructural damage modulates cortical activation in elderly subjects with MCI and leuko-araiosis. A DTI and fMRI study. Human Brain Mapping, 35(3), 819–830. https://doi.org/10.1002/hbm.22216.
Mascalchi, M., Pantoni, L., Giannelli, M., Valenti, R., Bianchi, A., Pracucci, G., Orsolini, S., Ciulli, S., Tessa, C., Poggesi, A., Pescini, F., Inzitari, D., & Diciotti, S. (2017). Diffusion tensor imaging to map brain microstructural changes in CADASIL. Journal of Neuroimaging, 27(1), 85–91. https://doi.org/10.1111/jon.12374.
Mascalchi, M., Tessa, C., Moretti, M., Della Nave, R., Boddi, V., Martini, S., Inzitari, D., & Villari, N. (2002). Whole brain apparent diffusion coefficient histogram: A new tool for evaluation of leukoaraiosis. Journal of Magnetic Resonance Imaging, 15(2), 144–148.
Measso, G., Cavarzeran, F., Zappalà, G., Lebowitz, B. D., Crook, T. H., Pirozzolo, F. J., Amaducci, L. A., Massari, D., & Grigoletto, F. (1993). The mini-mental state examination: Normative study of an Italian random sample. Developmental Neuropsychology, 9(2), 77–85. https://doi.org/10.1080/87565649109540545.
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
O'Sullivan, M., Morris, R. G., Huckstep, B., Jones, D. K., Williams, S. C., & Markus, H. S. (2004a). Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. Journal of Neurology, Neurosurgery, and Psychiatry, 75(3), 441–447.
O'Sullivan, M., Singhal, S., Charlton, R., & Markus, H. S. (2004b). Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia. Neurology, 62(5), 702–707.
O'Sullivan, M., Summers, P. E., Jones, D. K., Jarosz, J. M., Williams, S. C., & Markus, H. S. (2001). Normal-appearing white matter in ischemic leukoaraiosis: A diffusion tensor MRI study. Neurology, 57(12), 2307–2310.
Otsuka, Y., Yamauchi, H., Sawamoto, N., Iseki, K., Tomimoto, H., & Fukuyama, H. (2012). Diffuse tract damage in the hemispheric deep white matter may correlate with global cognitive impairment and callosal atrophy in patients with extensive leukoaraiosis. AJNR. American Journal of Neuroradiology, 33(4), 726–732. https://doi.org/10.3174/ajnr.A2853.
Pantoni, L., Basile, A. M., Pracucci, G., Asplund, K., Bogousslavsky, J., Chabriat, H., Erkinjuntti, T., Fazekas, F., Ferro, J. M., Hennerici, M., O’Brien, J., Scheltens, P., Visser, M. C., Wahlund, L. O., Waldemar, G., Wallin, A., & Inzitari, D. (2005). Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: Rationale, design and methodology. Neuroepidemiology, 24(1–2), 51–62. https://doi.org/10.1159/000081050.
Pasi, M., Salvadori, E., Poggesi, A., Ciolli, L., Del Bene, A., Marini, S., et al. (2015). White matter microstructural damage in small vessel disease is associated with Montreal cognitive assessment but not with mini mental state examination performances: Vascular mild cognitive impairment Tuscany study. Stroke, 46(1), 262–264. https://doi.org/10.1161/STROKEAHA.114.007553.
Pendlebury, S. T., Cuthbertson, F. C., Welch, S. J. V., Mehta, Z., & Rothwell, P. M. (2010). Underestimation of cognitive impairment by mini-mental state examination versus the Montreal cognitive assessment in patients with transient ischemic attack and stroke a population-based study. Stroke, 41(6), 1290–1293. https://doi.org/10.1161/Strokeaha.110.579888.
Poggesi, A., Salvadori, E., Pantoni, L., Pracucci, G., Cesari, F., Chiti, A., et al. (2012). Risk and Determinants of Dementia in Patients with Mild Cognitive Impairment and Brain Subcortical Vascular Changes: A Study of Clinical, Neuroimaging, and Biological Markers-The VMCI-Tuscany Study: Rationale, Design, and Methodology. Int J Alzheimers Dis, 2012, 608013, doi:10.1155/2012/608013.
Roman, G. C., Erkinjuntti, T., Wallin, A., Pantoni, L., & Chui, H. C. (2002). Subcortical ischaemic vascular dementia. Lancet Neurology, 1(7), 426–436.
Salvadori, E., Poggesi, A., Pracucci, G., Inzitari, D., & Pantoni, L. (2015). Development and psychometric properties of a neuropsychological battery for mild cognitive impairment with small vessel disease: The VMCI-Tuscany study. Journal of Alzheimer's Disease, 43(4), 1313–1323. https://doi.org/10.3233/JAD-141449.
Salvadori, E., Poggesi, A., Valenti, R., Pracucci, G., Pescini, F., Pasi, M., Nannucci, S., Marini, S., Del Bene, A., Ciolli, L., Ginestroni, A., Diciotti, S., Orlandi, G., Di Donato, I., De Stefano, N., Cosottini, M., Chiti, A., Federico, A., Dotti, M.T., Bonuccelli, U., Inzitari, D., Pantoni, L.; VMCI-Tuscany Study Group. (2016). Operationalizing mild cognitive impairment criteria in small vessel disease: The VMCI-Tuscany study. Alzheimers Dement, 12(4), 407–418. https://doi.org/10.1016/j.jalz.2015.02.010.
Schmahmann, J. D. (1996). From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping, 4(3), 174–198, doi:10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2–0. https://doi.org/10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. https://doi.org/10.1002/hbm.10062.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., Watkins, K. E., Ciccarelli, O., Cader, M. Z., Matthews, P. M., & Behrens, T. E. J. (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051.
Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061.
Stoodley, C. J., & Schmahmann, J. D. (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage, 44(2), 489–501. https://doi.org/10.1016/j.neuroimage.2008.08.039.
Tuladhar, A. M., van Norden, A. G., de Laat, K. F., Zwiers, M. P., van Dijk, E. J., Norris, D. G., et al. (2015). White matter integrity in small vessel disease is related to cognition. Neuroimage Clin, 7, 518–524. https://doi.org/10.1016/j.nicl.2015.02.003.
Vernooij, M. W., Ikram, M. A., Vrooman, H. A., Wielopolski, P. A., Krestin, G. P., Hofman, A., Niessen, W. J., van der Lugt, A., & Breteler, M. M. B. (2009). White matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry, 66(5), 545–553. https://doi.org/10.1001/archgenpsychiatry.2009.5.
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., Nordberg, A., Backman, L., Albert, M., Almkvist, O., Arai, H., Basun, H., Blennow, K., de Leon, M., DeCarli, C., Erkinjuntti, T., Giacobini, E., Graff, C., Hardy, J., Jack, C., Jorm, A., Ritchie, K., van Duijn, C., Visser, P., & Petersen, R. C. (2004). Mild cognitive impairment - beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. Journal of Internal Medicine, 256(3), 240–246. https://doi.org/10.1111/j.1365-2796.2004.01380.x.
Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060.
Wycoco, V., Shroff, M., Sudhakar, S., & Lee, W. (2013). White matter anatomy. what the radiologist needs to know. Neuroimaging Clin N Am, 23(2), 197–216. https://doi.org/10.1016/j.nic.2012.12.002.
Xu, Q., Cao, W. W., Mi, J. H., Yu, L., Lin, Y., & Li, Y. S. (2014). Brief screening for mild cognitive impairment in subcortical ischemic vascular disease: A comparison study of the Montreal cognitive assessment with the mini-mental state examination. European Neurology, 71(3–4), 106–114. https://doi.org/10.1159/000353988.
Xu, Q., Zhou, Y., Li, Y. S., Cao, W. W., Lin, Y., Pan, Y. M., et al. (2010). Diffusion tensor imaging changes correlate with cognition better than conventional MRI findings in patients with subcortical ischemic vascular disease. Dementia and Geriatric Cognitive Disorders, 30(4), 317–326. https://doi.org/10.1159/000320491.
Yuan, J. L., Wang, S. K., Guo, X. J., Teng, L. L., Jiang, H., Gu, H., & Hu, W. L. (2017). Disconnections of Cortico-subcortical pathways related to cognitive impairment in patients with Leukoaraiosis: A preliminary diffusion tensor imaging study. European Neurology, 78(1–2), 41–47. https://doi.org/10.1159/000477899.
Zhang, H., Avants, B. B., Yushkevich, P. A., Woo, J. H., Wang, S., McCluskey, L. F., et al. (2007). High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: An example study using amyotrophic lateral sclerosis. IEEE Transactions on Medical Imaging, 26(11), 1585–1597. https://doi.org/10.1109/TMI.2007.906784.
Funding
The VMCI-Tuscany was funded by Tuscany Region Health Programme in the framework of the “Bando Regione Salute 2009”.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary Fig. 1
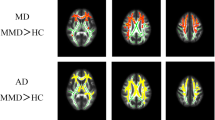
Between-group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute standard space) identifies in yellow WM tracts showing a significant (p-value <0.05 corrected, threshold-free cluster enhancement) increase of MD in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami, midbrain, pons, middle cerebellar peduncles and left cerebellar hemisphere. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 213 kb)
Supplementary Fig. 2
Between-group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) identifies in red WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) decrease of FA in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami, midbrain, pons, superior and middle cerebellar peduncles and cerebellar hemispheres. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 218 kb)
Supplementary Fig. 3
Between-group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) identifies in green WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) increase of RD in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami, midbrain, pons, superior and middle cerebellar peduncles and left cerebellar hemisphere. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 213 kb)
Supplementary Fig. 4
Between group TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) identifies in blu/light-blu WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) increase of AD in patients with vascular MCI and SVD as compared to healthy controls. They include WM tracts in the cerebral hemispheres, corpus callosum and thalami. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 206 kb)
Supplementary Fig. 5
Within-group regression TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) in patients with vascular MCI and SVD. Red identifies WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) positive correlation of FA with MoCA scores. They include WM tracts in the cerebral hemispheres, corpus callosum, and thalami. The spatial distribution of these WM tracts is bilateral but not symmetrical. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 159 kb)
Supplementary Fig. 6
Within-group regression TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) in patients with vascular MCI and SVD. Blue identifies the WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) negative correlation of axial diffusivity with MoCA scores. They include WM tracts in the cerebral hemispheres, corpus callosum, and left thalamus. The spatial distribution of these WM tracts is bilateral but not symmetrical. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 150 kb)
Supplementary Fig. 7
Within-group regression TBSS analysis at different anatomic levels (z coordinates in Montreal Neurological Institute space) in patients with vascular MCI and SVD. Green identifies the WM tracts showing a significant (p value <0.05 corrected, threshold-free cluster enhancement) negative correlation of RD with MoCA scores. They include WM tracts in the cerebral hemispheres, corpus callosum, thalami and decussation of superior cerebellar peduncles in the midbrain. The spatial distribution of these WM tracts is bilateral but not symmetrical. The red-yellow overlay shows the group FLAIR-lesion map. See text for abbreviations (GIF 157 kb)
Rights and permissions
About this article
Cite this article
Mascalchi, M., Salvadori, E., Toschi, N. et al. DTI-derived indexes of brain WM correlate with cognitive performance in vascular MCI and small-vessel disease. A TBSS study. Brain Imaging and Behavior 13, 594–602 (2019). https://doi.org/10.1007/s11682-018-9873-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9873-5