Abstract
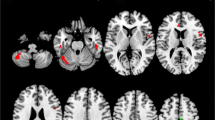
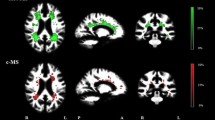
The corpus callosum (CC) is commonly affected in multiple sclerosis (MS), however, sensitive behavioral measures of MS-related CC pathology are lacking. The CC is considered a key structure in the mediation of a type of involuntary movement known as motor overflow. In this study, we sought to characterize the impact of CC damage on motor overflow in MS. Twenty MS participants and 20 controls performed a unilateral force production task. Motor overflow (involuntary force) in the non-active hand was measured while the active hand performed the task. CC volume and lesion load were calculated for MS participants using T2-weighted MRI. We found no group differences in motor overflow; however, motor overflow correlated significantly with MS disease severity [Expanded disability status scale (EDSS)]. CC damage (lesions and decreased volume) did not correlate with motor overflow. This study suggests that CC damage may not directly lead to changes in the regulation of motor overflow. Rather, findings support the notion that a wider network of structures may mediate the production and suppression of motor overflow.


Similar content being viewed by others
References
Filippi M, Rocca M (2005) MRI evidence for multiple sclerosis as a diffuse disease of the central nervous system. J Neurol 252(S5):v16–v24
Keegan BM, Noseworthy JH (2002) Multiple sclerosis. Annu Rev Med 53:285–302
Evangelou N, Konz D, Esiri MM et al (2000) Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 123:1845–1849
Ozturk A, Smith SA, Gordon-Lipkin EM et al (2010) MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler 16(2):166–177
Barnard RO, Triggs M (1974) Corpus callosum in multiple sclerosis. J Neurol Neurosurg Psychiatry 37(11):1259–1264
Ge Y, Law M, Johnson G et al (2004) Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 20(1):1–7
Lin X, Tench CR, Morgan PS et al (2005) ‘Importance sampling’ in MS: use of diffusion tensor tractography to quantify pathology related to specific impairment. J Neurol Sci 237:13–19
Lin X, Tench CR, Morgan PS et al (2008) Use of combined and conventional quantitative MRI to quantify pathology related to cognitive impairment in multiple sclerosis. J Neurol Neurosurg Psychiatry 79:437–441
Larson EB, Burnison DS, Brown WS (2002) Callosal function in multiple sclerosis: bimanual motor coordination. Cortex 38(2):201–214
Pelletier J, Habib M, Lyoncaen O et al (1993) Functional and magnetical-resonance-imaging correlates of callosal involvement in multiple sclerosis. Arch Neurol 50(10):1077–1082
Pelletier J, Suchet L, Witjas T et al (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol 58(1):105–111
Sigal T, Shmuel M, Mark D et al (2012) Diffusion tensor imaging of corpus callosum integrity in multiple sclerosis: correlation with disease variables. J Neuroimaging 22(1):33–37
Brown LN, Zhang Y, Mitchell JR et al (2012) Corpus callosum volume and interhemispheric transfer in multiple sclerosis. Can J Neurol Sci 37:615–619
Barkhof F, Tas M, Valk J et al (1998) Functional correlates of callosal atrophy in relapsing-remitting multiple sclerosis patients. A preliminary MRI study. J Neurol 245(3):153–158
Manson S, Palace J, Frank JA et al (2006) Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res 174(4):728–733
Lenzi D, Conte A, Mainero C et al (2007) Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp 28(7):636–644
Hoppner J, Kunesch E, Buchmann J et al (1999) Demyelination and axonal degeneration in corpus callosum assessed by analysis of transcallosally mediated inhibition in multiple sclerosis. Clin Neurophysiol 110(4):748–756
Lee M, Reddy H, Johansen-Berg H et al (2000) The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol 47(5):606–613
Hubers A, Orekhov Y, Ziemann U (2008) Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity. Eur J Neurosci 28(2):364–371
Giovannelli F, Borgheresi A, Balestrieri F et al (2009) Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol (London) 587(22):5393–5410
Hoy KE, Fitzgerald PB, Bradshaw JL et al (2004) Investigating the cortical origins of motor overflow. Brain Res Rev 46:315–327
Armatas CA, Summers JJ, Bradshaw JL (1994) Mirror movements in normal adult subjects. J Clin Exp Neuropsychol 16(3):405–412
Georgiou-Karistianis N, Hoy KE, Bradshaw JL et al (2004) Motor overflow in Huntington’s disease. J Neurol Neurosurg Psychiatry 75:904–906
Hoy KE, Fitzgerald PB, Bradshaw JL et al (2004) Motor overflow in Schizophrenia. Psychiatry Res 125:129–137
Hoy KE, Georgiou-Karistianis N, Laycock R et al (2007) Using transcranial magnetic stimulation to investigate the cortical origins of motor overflow: a study in schizophrenia and healthy controls. Psychol Med 37:583–594
Addamo PK, Farrow M, Hoy KE et al (2009) A developmental study of the influence of task characteristics on motor overflow. Brain Cogn 69:413–419
Addamo PK, Farrow M, Hoy KE et al (2009) The influence of task characteristics on younger and older adult motor overflow. Q J Exp Psychol 62(2):239–247
Mayston M, Harrison L, Stephens J (1999) A neurophysiological study of mirror movements in adults and children. Ann Neurol 45(5):583–594
Fujiyama H, Garry MI, Levin O et al (2009) Age-related differences in inhibitory processes during interlimb coordination. Brain Res 1262:38–47
Bartels C, Mertens N, Hofer S et al (2008) Callosal dysfunction in amyotrophic lateral sclerosis correlates with diffusion tensor imaging of the central motor system. Neuromuscul Disord 18:398–407
McDonald W, Compston A, Edan G et al (2001) Recommended diagnostic criteria for multiple sclerosis; guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Oldfield RC (1971) The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113
Nelson HE (1982) National adult reading test: test manual. NFER-Nelson, Windsor
Armatas CA, Summers JJ, Bradshaw JL (1996) Handedness and performance variability as factors influencing mirror movement occurrence. J Clin Exp Neuropsychol 18(6):823–835
Beck A, Steer RA, Brown GK (1996) Beck depression inventory-second edition manual. The Psychological Corporation, San Antonio TX
Armatas CA, Summers JJ, Bradshaw JL (1996) Strength as a factor influencing mirror movements. Hum Mov Sci 492:1–17
Miller DH, Barkhof F, Frank JA et al (2002) Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125(8):1676–1695
Ge Y (2006) Multiple sclerosis: the role of MR imaging. Am J Neuroradiol 27:1165–1176
Talairach J, Torneauz P (1993) Referentially oriented cerebral MRI anatomy: Atlas of stereotaxic anatomical correlations for gray and white matter. Thieme Medical Publishers Inc., New York
Anstey KJ, Mack HA, Christensen H et al (2007) Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia 45(8):1911–1920
Shrout P, Fleiss JL (1979) Intraclass correlation: uses in assessing rater reliability. Psychol Bull 86:420–428
Witelson SF (1989) Hand and sex differences in the isthmus and genu of the human corpus callosum. A post-mortem morphological study. Brain 112(3):799–835
Beauchamp MH, Anderson VA, Catroppa C et al (2009) Implications of reduced callosal area for social skills after severe traumatic brain injury in children. J Neurotrauma 26(10):1645–1654
Panizzon MS, Hoff AL, Nordahl TE et al (2003) Sex differences in the corpus callosum of patients with schizophrenia. Schizophr Res 62(1–2):115–122
Sun J, Maller JJ, Daskalakis ZJ et al (2009) Morphology of the corpus callosum in treatment-resistant schizophrenia and major depression. Acta Psychiatr Scand 120(4):265–273
Pasini A, D’agati E (2009) Pathophysiology of NSS in ADHD. World J Bio Psychiatry 10(4–2):495–502
Brinkman C (1984) Supplementary motor area of the monkey’s cerebral cortex: short and long term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci 4(4):918–929
Weiller C, Ramsay SC, Wise RJS et al (1993) Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol 33:181–189
Lepage J-F, Beaule V, Srour M et al (2012) Neurophysiological investigation of congenital mirror movements in a patient with agenesis of the corpus callosum. Brain Stimul 5:137–140
Meyer B-U, Roricht S, von Einsiedel HG et al (1995) Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118(2):429–440
Uttner I, Mai N, Esslinger O et al (2005) Quantitative evaluation of mirror movements in adults with focal brain lesions. Eur J Neurol 12(12):964–975
Ranjeva JP, Audoin B, Duong MVA et al (2005) Local tissue damage assessed with statistical mapping analysis of brain magnetization transfer ratio: relationship with functional status of patients in the earliest stage of multiple sclerosis. Am J Neuroradiol 26(1):119–127
Acknowledgments
Supported by research funds from the School of Psychology and Psychiatry, Monash University.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ternes, AM., Maller, J.J., Fielding, J. et al. Investigating the role of the corpus callosum in regulating motor overflow in multiple sclerosis. J Neurol 260, 1997–2004 (2013). https://doi.org/10.1007/s00415-013-6914-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-6914-2