Abstract
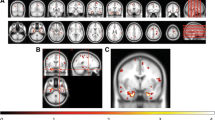
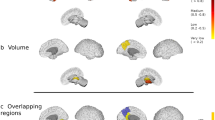
We investigated the patterns of regional distribution of focal lesions, white matter (WM) and gray matter (GM) atrophy in patients with cortical (cort) MS in comparison to classical (c) MS patients. Nine cort-MS, nine c-MS and nine age-matched healthy controls (HC) underwent a brain MRI exam, including FLAIR and high-resolution T1-weighted scans. MS patients underwent neurological and neuropsychological assessment. Between-group differences of GM and WM volumes and their correlations with neuropsychological performances were assessed with voxel-based morphometry. FLAIR and T1 lesion probability maps (LPMs) were also obtained. Performance at neuropsychological tests was worse in cort-MS than in c-MS patients. Compared to HC, MS patients had a distributed pattern of GM and WM atrophy. No GM/WM area was more atrophic in c-MS vs cort-MS patients. Compared to c-MS, cort-MS patients experienced GM atrophy of frontal–temporal–parietal areas and cingulate cortex and WM atrophy of the cingulum bundle, bilateral cerebral peduncles, right inferior longitudinal fasciculus and left superior longitudinal fasciculus. FLAIR and T1 LPMs did not differ between c-MS vs cort-MS patients. A higher susceptibility to neurodegenerative processes in key brain regions known to be related to cognitive functions is likely to underlie the clinical manifestations of cort-MS.



Similar content being viewed by others
References
Herderschee D, Stam J, Derix MM (1987) Aphemia as a first symptom of multiple sclerosis. J Neurol Neurosurg Psychiatry 50:499–500
Achiron A, Ziv I, Djaldetti R, Goldberg H, Kuritzky A, Melamed E (1992) Aphasia in multiple sclerosis: clinical and radiologic correlations. Neurology 42:2195–2197
Skegg K (1993) Multiple sclerosis presenting as a pure psychiatric disorder. Psychol Med 23:909–914
Zarei M, Chandran S, Compston A, Hodges J (2003) Cognitive presentation of multiple sclerosis: evidence for a cortical variant. J Neurol Neurosurg Psychiatry 74:872–877
Zarei M (2006) Clinical characteristics of cortical multiple sclerosis. J Neurol Sci 245:53–58
Benedict RH, Carone DA, Bakshi R (2004) Correlating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosis. J Neuroimaging 14:36S–45S
Morgen K, Sammer G, Courtney SM, Wolters T, Melchior H, Blecker CR, Oschmann P et al (2006) Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing–remitting MS. Neuroimage 30:891–898
Riccitelli G, Rocca MA, Pagani E, Rodegher ME, Rossi P, Falini A, Comi G et al (2011) Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum Brain Mapp 32:1535–1543
Orme JE (1961) The Coloured Progressive Matrices as a measure of intellectual subnormality. Br J Med Psychol 34:291–292
De Renzi E, Vignolo LA (1962) The token test: a sensitive test to detect receptive disturbances in aphasics. Brain 85:665–678
Novelli GPC, Capitani E, Laiacona M, Cappa SF, Vallar G (1986) Tre test clinici di memoria verbale a lungo termine. Taratura su soggetti normali. Archivio di Psicologia Neurologia e Psichiatria 2:278–296
Spinnler HTG (1987) Standardizzazione e taratura italiana di test psicometrici. Ital J Neurol Sci 8:7–120
Orsini AGD, Capitani E, Laiacona M, Papagno C, Vallar G (1987) Verbal and spatial immediate memory span: normative data from 1355 adults and 112 children. Ital J Neurol Sci 8:539–548
Amato MP, Portaccio E, Goretti B, Zipoli V, Ricchiuti L, De Caro MF, Patti F et al (2006) The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12:787–793
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CA (2010) Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging 32:223–228
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Mori S, Wakana S, Van Zijl PCM, Nagae-Poetscher LM (2005) MRI atlas of human white matter. Elsevier, Amsterdam
Metzler-Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O’Sullivan MJ (2012) Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J Neurosci: Off J Soc Neurosci 32:17612–17619
Villain N, Desgranges B, Viader F, de la Sayette V, Mezenge F, Landeau B, Baron JC et al (2008) Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer’s disease. J Neurosci: Off J Soc Neurosci 28:6174–6181
Villain N, Fouquet M, Baron JC, Mezenge F, Landeau B, de La Sayette V, Viader F et al (2010) Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain 133:3301–3314
Zhang S, Li CS (2014) Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect 4:53–69
Davachi L, Maril A, Wagner AD (2001) When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. J Cogn Neurosci 13:1059–1070
Stoeckel C, Gough PM, Watkins KE, Devlin JT (2009) Supramarginal gyrus involvement in visual word recognition. Cortex 45:1091–1096
Westerhausen R, Kompus K, Hugdahl K (2014) Mapping hemispheric symmetries, relative asymmetries, and absolute asymmetries underlying the auditory laterality effect. Neuroimage 84:962–970
Nagels A, Kauschke C, Schrauf J, Whitney C, Straube B, Kircher T (2013) Neural substrates of figurative language during natural speech perception: an fMRI study. Front Behav Neurosci 7:121
Tanaka S, Honda M, Sadato N (2005) Modality-specific cognitive function of medial and lateral human Brodmann area 6. J Neurosci: Off J Soc Neurosci 25:496–501
Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9:856–869
Fox CJ, Iaria G, Barton JJ (2008) Disconnection in prosopagnosia and face processing. Cortex 44:996–1009
ffytche DH, Catani M (2005) Beyond localization: from hodology to function. Philos Trans R Soc Lond B Biol Sci 360:767–779
Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP et al (2012) White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull 38:1308–1317
Lebel C, Beaulieu C (2009) Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30:3563–3573
Robertson IH (2013) A noradrenergic theory of cognitive reserve: implications for Alzheimer’s disease. Neurobiol Aging 34:298–308
Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR et al (2009) Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. Neuroimage 45:10–16
Conflicts of interests
Laura Parisi, Flavia Mattioli, Ruggero Capra, Chiara Stampatori, and Fabio Bellomi report no disclosures.
Maria A. Rocca received speakers honoraria from Biogen Idec, Novartis and Serono Symposia International Foundation and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla.
Massimo Filippi serves on scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, and the Jacques and Gloria Gossweiler Foundation (Switzerland).
Ethical standard
This study was approved by the Local Ethical Committes on human studies and written informed consent from each subject was obtained prior to their enrolment.
Statement of Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parisi, L., Rocca, M.A., Mattioli, F. et al. Patterns of regional gray matter and white matter atrophy in cortical multiple sclerosis. J Neurol 261, 1715–1725 (2014). https://doi.org/10.1007/s00415-014-7409-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7409-5