Abstract
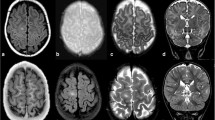
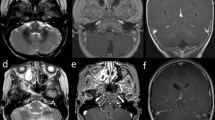
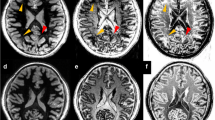
Cortical tubers are very common in tuberous sclerosis complex (TSC) and widely vary in size, appearance and location. The relationship between tuber features and clinical phenotype is unclear. The aim of the study is to propose a classification of tuber types along a spectrum of severity, using magnetic resonance imaging (MRI) characteristics in 35 patients with TSC and history of epilepsy, and to investigate the relationship between tuber types and genetics, as well as clinical manifestations. Three types of tubers were identified based on the MRI signal intensity of their subcortical white matter component. (1) Tubers Type A are isointense on volumetric T1 images and subtly hyperintense on T2 weighted and fluid-attenuated inversion recovery (FLAIR); (2) Type B are hypointense on volumetric T1 images and homogeneously hyperintense on T2 weighted and FLAIR; (3) Type C are hypointense on volumetric T1 images, hyperintense on T2 weighted, and heterogeneous on FLAIR characterized by a hypointense central region surrounded by a hyperintense rim. Based on the dominant tuber type present, three distinct patient groups were also identified: Patients with Type A tuber dominance have a milder phenotype. Patients with Type C tuber dominance have more MRI abnormalities such as subependymal giant cell tumors, and were more likely to have an autism spectrum disorder, a history of infantile spasms, and a higher frequency of epileptic seizures, compared to patients who have a dominance in Type B tubers, and especially to those with a Type A dominance.




Similar content being viewed by others
References
Roach ES, Sparagana SP (2004) Diagnosis of tuberous sclerosis complex. J Child Neurol 19:643–649
Bozzao A, Manenti G, Curatolo P (2003) Neuroimaging. In: Curatolo P (ed) Tuberous sclerosis complex: from basic science to clinical phenotypes. Mac Keith Press for the International Child Neurology Association, London, pp 109–123
DiMario FJ (2004) Brain abnormalities in tuberous sclerosis complex. J Child Neurol 19:650–657
Griffiths PD, Bolton P, Verity C (1998) White matter abnormalities in tuberous sclerosis complex. Acta Radiol 39:482–486
Ridler K, Suckling J, Higgins N, Bolton P, Bullmore E (2004) Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex. J Child Neurol 19:658–665
Crino PB, Trojanowski JQ, Dichter MA, Eberwine J (1996) Embryonic neuronal markers in TS single-cell molecular pathology. Proc Natl Acad Sci USA 93:14152–14157
Hirose T, Schelthauer BW, Lopes MBS, Gerber HA, Altermatt HJ, Hukee MJ et al (1995) Tuber and subependymal giant cell astrocytoma associated with tuberous sclerosis: an immunohistochemical, ultrastructural, and immunoelectron microscopic study. Acta Neuropathol 90:387–399
Braffman BH, Bilaniuk LT, Naidich TP, Altman NR, Post MJ, Quencer RM et al (1992) MR imaging of tuberous sclerosis: pathogenesis of the phakomatosis, use of gadopentetate dimeglumine, and literature review. Radiology 183:227–238
Suronov AA, Wu X, Weiner HL, Mikell CB, Goodman RR, Crino PD et al (2008) Tuberous sclerosis: a primary pathology of astrocytes? Epilepsia 49:53–62
Trombley IK, Mirra SS (1981) Ultrastructure of tuberous sclerosis: cortical tuber and subpendymal tumor. Ann Neurol 9:174–181
Stefansson K, Wollman RL, Huttenlocher PR (1999) Lineage of cells in the central nervous system. In: Gomez MR, Sampson JR, Whittemore VH (eds) Tuberous sclerosis complex, 3rd edn. Oxford University Press, New York, pp 251–262
Doherty C, Goh S, Young Poussaint T, Erdag N, Thiele EA (2005) Prognostic significance of tuber count and location in tuberous sclerosis complex. J Child Neurol 20:837–841
Pinto Gama HP, da Rocha AJ, Braga FT, da Silva CJ, Martins Maia AC Jr, de Campos Meirelles RG et al (2006) Comparative analysis of MR sequences to detect structural brain lesions in tuberous sclerosis. Pediatr Radiol 36:119–125
Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355:1345–1356
Doherty C, Goh S, Poussaint TY, Erdag N, Thiele EA (2006) Prognostic significance of tuber count and location in tuberous sclerosis complex. J Child Neurol 20:837–841
Jansen FE, Vincken KL, Algra A, Anbeek P, Braams O, Nellist M et al (2008) Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology 70:916–923
O’Callaghan FJ, Harris T, Joinson C, Botton P, Noakes M, Presdee D et al (2004) The relation of infantile spasms, tubers, and intelligence in tuberous sclerosis complex. Arch Dis Child 89:530–533
Schwartz RA, Fernandez G, Kotulska K, Jozwiak S (2007) Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol 57:189–202
Asano E, Chugani DC, Muzik O, Shen C, Juhász C, Janisse J et al (2000) Multimodality imaging for improved detection of epileptogenic foci in tuberous sclerosis complex. Neurology 54:1976–1984
Chugani DC, Chugani HT, Muzik O, Shah JR, Shah AK, Canady A et al (1998) Imaging epileptogenic tuber in children with tuberous sclerosis complex using α-[11C]methyl-l-tryptophan positron emission tomography. Ann Neurol 44:858–866
Fedi M, Reutens DC, Andermann F, Okazawa H, Boling W, White C et al (2003) alpha-[11C]-Methyl-l-tryptophan PET identifies the epileptogenic tuber and correlates with interictal spike frequency. Epilepsy Res 52:203–213
Jansen FE, Braun KPJ, van Nieuwenhuizen O, Huiskamp G, Vincken KL, van Huffelen AC et al (2003) Diffusion-weighted magnetic resonance imaging and identification of the epileptogenic tuber in patient with tuberous sclerosis. Arch Neurol 60:1580–1584
Major P, Rakowski S, Simon MV, Cheng ML, Eskandar E, Baron J et al (2009) Are cortical tubers epileptogenic? Evidence from electrocorticography. Epilepsia 50:147–154
Pellizzi GB (1901) Contnibuto allo studio dell’idiozia: rivisita sperimentale di freniatnia e medicine legate delle alienazioni mental. Riv Sper Freniatr 27:265–269
Chu-shore CJ, Major P, Montenegro M, Thiele EA (2009) Cyst-like tubers are associated with TSC2 and epilepsy in tuberous sclerosis complex. Neurology 72:1165–1169
Marti-Bonmati L, Menor F, Dosda R (2000) Tuberous sclerosis: differences between cerebral and cerebellar cortical tubers in a pediatric population. AJNR Am J Neuroradiol 21:557–560
Jurkiewicz E, Jozwiak S, Bekiesinska-Figatowska M, Papula-Kosciesza I, Walecki J (2006) Cyst-like cortical tubers in patients with tuberous sclerosis complex: MR imaging with the FLAIR sequence. Pediatr Radiol 36:498–501
Firat AK, Karakas HM, Erdem G, Yakinci C, Bicak U (2006) Diffusion weighted MR findings of brain involvement in tuberous sclerosis. Diagn Interv Radiol 12:57–60
Goh S, Butler W, Thiele EA (2004) Subpendymal giant cell tumors in tuberous sclerosis complex. Neurology 63:1457–1461
Shepherd CW, Gomez MR, Lie JT, Crowson CS (1991) Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 66:792–796
Goodman M, Lamm SH, Engel A, Sheperd CW, Houser OW, Gomez MR (1997) Cortical tuber counts: a biomarker indicating neurologic severity of tuberous sclerosis complex. J Child Neurol 21:85–90
Makki MI, Chugani DC, Janisse J, Chugani HT (2007) Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol 28:1662–1667
Sener RN (2002) Tuberous sclerosis: diffusion MRI findings in the brain. Eur Radiol 12:138–143
Chandra PS, Salamon N, Huang J, Wu JY, Koh S, Vinters HV et al (2006) FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia 47:1543–1549
Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR et al (2007) Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 9:88–100
Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS et al (2001) Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared to TSC1, disease in multiple organs. Am J Hum Genet 68:64–80
Jansen FE, Braams O, Vincken KL, Algra A, Anbeek P, Jennekens-Schinkel A et al (2008) Overlapping neurologic and cognitive phenotypes in patients with TSC1 or TSC2 mutations. Neurology 70:908–915
Strizhera GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP (2001) The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Resp Crit Care Med 163:253–258
de Vries PJ, Howe CJ (2007) The tuberous sclerosis complex proteins—a GRIPP on cognition and neurodevelopment. Trends Mol Med 13:319–326
Joinson C, O’Callaghan FJK, Osborne JP, Martyn C, Harris T, Bolton PF (2003) Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis. Psychol Med 33:335–344
Winterkorne EB, Pulsifer MB, Thiele EA (2007) Cognitive prognosis of patients with tuberous sclerosis complex. Neurology 68:62–64
Gomez MR, Sampson JR, Whittemore VH (1999) Tuberous sclerosis complex, 3rd edn. Oxford University Press, New York
Goh S, Kwiatkowski DJ, Dorer DJ, Thiele EA (2005) Infantile spasms and intellectual outcomes in children with tuberous sclerosis complex. Neurology 65:235–238
Jozwiak S, Goodman M, Lamm SH (1998) Poor mental development in patients with tuberous sclerosis complex. Arch Neurol 55:379–384
Curatolo P, Seri S, Verdecchia M, Bomnardieri R (2001) Infantile spasms in tuberous sclerosis complex. Brain Dev 23:502–507
Acknowledgments
We are grateful to Miguel Chagnon, Adam Numis and Larry MacDonald for providing advice and information regarding statistical analysis. This work was supported by a scholarship by the Canadian Institutes of Health Research (CIHR), awarded to Anne Gallagher, Ph.D. as well as by the Carol and James Herscot Center for Tuberous Sclerosis Complex.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallagher, A., Grant, E.P., Madan, N. et al. MRI findings reveal three different types of tubers in patients with tuberous sclerosis complex. J Neurol 257, 1373–1381 (2010). https://doi.org/10.1007/s00415-010-5535-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5535-2