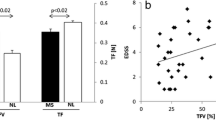
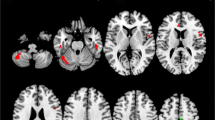
Abstract
Neuroimaging studies in amyotrophic lateral sclerosis (ALS) investigating movements of the hands have in general found increased activation compared to healthy controls, which has been interpreted in terms of cortical adaptation as a result of corticospinal tract damage. Here, we investigated brain activations to vertical tongue movements using functional MRI at 3 tesla. Whereas healthy controls, patients with Kennedy syndrome, and ALS patients without bulbar involvement showed robust and indistinguishable activations in pre- and postcentral areas and the thalamus, ALS patients with bulbar involvement showed a significant decrease of cortical activity and missing thalamic activity. This decrease stands in marked contrast to the increase of activity observed in ALS patients when performing limb movements. We discuss these divergent findings with regard to the different physiological properties of tongue and limb movements. These findings may also help to explain the faster time-course of the disease in patients with bulbar involvement.

Similar content being viewed by others
References
Alkadhi H, Crelier GR, Boendermaker SH, Golay X, Hepp-Reymond MC, Kollias SS (2002) Reproducibility of primary motor cortex somatotopy under controlled conditions. AJNR Am J Neuroradiol 23:1524–1532
Armon C, Moses D (1998) Linear estimates of rates of disease progression as predictors of survival in patients with ALS entering clinical trials. J Neurol Sci 160 Suppl 1:S37–S41
Bailey EF, Rice AD, Fuglevand AJ (2007) Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 97:933–936
Boynton GM, Engel SA, Glover GH, Heeger DJ (1996) Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16:4207–4221
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Cappellari A, Brioschi A, Barbieri S, Braga M, Scarlato G, Silani V (1999) A tentative interpretation of electromyographic regional differences in bulbar- and limb-onset ALS. Neurology 52:644–646
Chio A, Mora G, Leone M, Mazzini L, Cocito D, Giordana MT, Bottacchi E, Mutani R (2002) Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology 59:99–103
Coope S (1953) Muscle spindles in the intrinsic muscles of the human tongue. J Physiol 122:193–202
Cudkowicz ME, Shefner JM, Schoenfeld DA, Brown RH Jr, Johnson H, Qureshi M, Jacobs M, Rothstein JD, Appel SH, Pascuzzi RM, Heiman-Patterson TD, Donofrio PD, David WS, Russell JA, Tandan R, Pioro EP, Felice KJ, Rosenfeld J, Mandler RN, Sachs GM, Bradley WG, Raynor EM, Baquis GD, Belsh JM, Novella S, Goldstein J, Hulihan J (2003) A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology 61:456–464
Czaplinski A, Yen AA, Appel SH (2006) Amyotrophic lateral sclerosis: early predictors of prolonged survival. J Neurol 253:1428–1436
Czaplinski A, Yen AA, Simpson EP, Appel SH (2006) Predictability of disease progression in amyotrophic lateral sclerosis. Muscle Nerve 34:702–708
Davidoff RA (1990) The pyramidal tract. Neurology 40:332–339
del Aguila MA, Longstreth WT Jr, McGuire V, Koepsell TD, van Belle G (2003) Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology 60:813–819
Dziewas R, Teismann IK, Suntrup S, Schiffbauer H, Steinstraeter O, Warnecke T, Ringelstein EB, Pantev C (2008) Cortical compensation associated with dysphagia caused by selective degeneration of bulbar motor neurons. Hum Brain Mapp (Epub ahead of print)
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878
Havel P, Braun B, Rau S, Tonn JC, Fesl G, Bruckmann H, Ilmberger J (2006) Reproducibility of activation in four motor paradigms. An fMRI study. J Neurol 253:471–476
Haverkamp LJ, Appel V, Appel SH (1995) Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 118(Pt 3):707–719
Hesselmann V, Sorger B, Lasek K, Guntinas-Lichius O, Krug B, Sturm V, Goebel R, Lackner K (2004) Discriminating the cortical representation sites of tongue and up movement by functional MRI. Brain Topogr 16:159–167
Iwanaga K, Hayashi S, Oyake M, Horikawa Y, Hayashi T, Wakabayashi M, Kondo H, Tsuji S, Takahashi H (1997) Neuropathology of sporadic amyotrophic lateral sclerosis of long duration. J Neurol Sci 146:139–143
Kaufmann P, Levy G, Thompson JL, Delbene ML, Battista V, Gordon PH, Rowland LP, Levin B, Mitsumoto H (2005) The ALSFRSr predicts survival time in an ALS clinic population. Neurology 64:38–43
Kew JJ, Brooks DJ, Passingham RE, Rothwell JC, Frackowiak RS, Leigh PN (1994) Cortical function in progressive lower motor neuron disorders and amyotrophic lateral sclerosis: a comparative PET study. Neurology 44:1101–1110
Kew JJ, Leigh PN, Playford ED, Passingham RE, Goldstein LH, Frackowiak RS, Brooks DJ (1993) Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain 116(Pt 3):655–680
Kollewe K, Mauss U, Krampfl K, Petri S, Dengler R, Mohammadi B (2008) ALSFRS-R score and its ratio: a useful predictor for ALS-progression. J Neurol Sci 275:69–73
Konrad C, Henningsen H, Bremer J, Mock B, Deppe M, Buchinger C, Turski P, Knecht S, Brooks B (2002) Pattern of cortical reorganization in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Exp Brain Res 143:51–56
Konrad C, Jansen A, Henningsen H, Sommer J, Turski PA, Brooks BR, Knecht S (2006) Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172:361–369
Kubota K, Negishi T, Masegi T (1975) Topological distribution of muscle spindles in the human tongue and its significance in proprioception. Bull Tokyo Med Dent Univ 22:235–242
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V (1996) Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis/riluzole study group II. Lancet 347:1425–1431
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131
Louwerse ES, Visser CE, Bossuyt PM, Weverling GJ (1997) Amyotrophic lateral sclerosis: mortality risk during the course of the disease and prognostic factors. The Netherlands ALS Consortium. J Neurol Sci 152 Suppl 1:S10–S17
Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV (2002) Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve 25:709–714
Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS (2004) Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol 92:2428–2443
Porter R (1966) Lingual mechanoreceptors activated by muscle twitch. J Physiol 183:101–111
Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, Buchel C, Weiller C (2004) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350
Schoenfeld MA, Tempelmann C, Gaul C, Kuhnel GR, Duzel E, Hopf JM, Feistner H, Zierz S, Heinze HJ, Vielhaber S (2005) Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252:944–952
Stambler N, Charatan M, Cedarbaum JM (1998) Prognostic indicators of survival in ALS. ALS CNTF treatment study group. Neurology 50:66–72
Stanton BR, Williams VC, Leigh PN, Williams SC, Blain CR, Jarosz JM, Simmons A (2007) Altered cortical activation during a motor task in ALS. Evidence for involvement of central pathways. J Neurol 254:1260–1267
Stippich C, Blatow M, Durst A, Dreyhaupt J, Sartor K (2007) Global activation of primary motor cortex during voluntary movements in man. Neuroimage 34:1227–1237
Teismann IK, Steinstraeter O, Warnecke T, Pantev C, Ringelstein EB, Dziewas R (2008) Kortikale Schluckverarbeitung bei ALS Patienten mit rasch progredienter Dysphagie. Akt Neurologie 35:S112–S113
Urban PP, Wicht S, Hopf HC (2001) Sensitivity of transcranial magnetic stimulation of cortico-bulbar versus cortico-spinal tract involvement in amyotrophic lateral sclerosis (ALS). J Neurol 248:850–855
Acknowledgment
Supported by grants from the BMBF and the DFG to TFM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadi, B., Kollewe, K., Samii, A. et al. Decreased brain activation to tongue movements in amyotrophic lateral sclerosis with bulbar involvement but not Kennedy syndrome. J Neurol 256, 1263–1269 (2009). https://doi.org/10.1007/s00415-009-5112-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5112-8