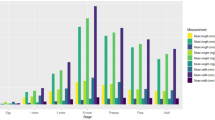
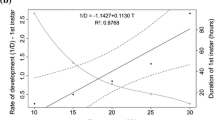
Abstract
Forensic entomology requires knowledge of the developmental rates of the species that colonize a body after death to estimate the postmortem interval (PMI). These developmental rates may vary depending not only on the species but also on the geographic location due to population differences. Therefore, the objectives of this work were to determine the developmental duration of the forensically important fly Chrysomya megacephala under constant controlled and field condition temperatures and to compare these results, through a meta-analysis, with data reported by other authors on populations from different localities. For this, C. megacephala colonies were established in the laboratory, and the duration of the life cycle was studied at two controlled temperatures (25 °C and 27 °C) and field conditions (27.5 ± 3.2 °C). Analysis of variance was performed to determine differences in developmental time and larval length between constant laboratory temperatures and field conditions. A generalized linear model was performed with predictor variables extracted from the literature (diet, relative humidity, latitude, longitude) to evaluate the effect of population variation on developmental times. The results showed significant differences in developmental times between 25 and 27 °C. As expected, the complete life cycle of C. megacephala was shorter at 27 °C. Finally, the meta-analysis suggested differences between the developmental times of different populations, based on temperature and geographic location. The results of this study provide fundamental developmental data to use C. megacephala in PMI estimations. Finally, we suggest that, when making expert reports, information from local populations should be used to determine a more accurate and reliable PMI.



Similar content being viewed by others
References
Pittner S, Bugelli V, Weitgasser K et al (2020) A field study to evaluate PMI estimation methods for advanced decomposition stages. Int J Leg Med 134:1361–1373. https://doi.org/10.1007/s00414-020-02278-0
Wells JD, LaMotte LR (2010) Estimating the postmortem interval. In: Byrd JH, Tomberlin J (eds) Forensic entomology: the utility of arthropods in legal investigations, 3rd edn. CRC Press, Boca Raton, FL, pp 213–224
Shrestha R, Kanchan T, Krishan K (2022) Methods of estimation of time since death. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023
Campobasso CP, Vella GD, Introna F (2001) Factors affecting decomposition and Diptera colonization. Forensic Sci Int 120:18–27. https://doi.org/10.1016/S0379-0738(01)00411-X
Amendt J, Richards CS, Campobasso CP et al (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol 7:379–392. https://doi.org/10.1007/s12024-010-9209-2
Sharma R, Garg RK, Gaur JR (2015) Various methods for the estimation of the post mortem interval from Calliphoridae: a review. Egypt J Forensic Sci 5:1–12. https://doi.org/10.1016/j.ejfs.2013.04.002
Lei G, Liu F, Liu P et al (2019) A bibliometric analysis of forensic entomology trends and perspectives worldwide over the last two decades (1998–2017). Forensic Sci Int 295:72–82. https://doi.org/10.1016/j.forsciint.2018.12.002
Lutz L, Zehner R, Verhoff MA et al (2021) It is all about the insects: a retrospective on 20 years of forensic entomology highlights the importance of insects in legal investigations. Int J Legal Med 135:2637–2651. https://doi.org/10.1007/s00414-021-02628-6
Matuszewski S (2021) Post-mortem interval estimation based on insect evidence: current challenges. Insects 12:314–334. https://doi.org/10.3390/insects12040314
Catts EP, Goff ML (1992) Forensic entomology in criminal investigations. Annu Rev Entomol 37:253–272
Archer MS (2004) Annual variation in arrival and departure times of carrion insects at carcasses: implications for succession studies in forensic entomology. Aust J Zool 51:569–576. https://doi.org/10.1071/ZO03053
Anderson GS (2000) Insect succession on carrion and its relationship to determining time of death. In: Byrd JH, Tomberlin J (eds) Forensic entomology: the utility of arthropods in legal investigations, 3rd edn. CRC Press, Boca Raton, FL, pp 143–175
Acosta X, González-Reyes AX, Corronca JA et al (2021) Estimation of the postmortem interval through the use of development time of two South American Species of forensic importance of the genus Lucilia (Diptera: Calliphoridae). J Med Entomol 58:1064–1073. https://doi.org/10.1093/jme/tjab001
Wang M, Wang Y, Hu G et al (2020) Development of Lucilia sericata (Diptera: Calliphoridae) under constant temperatures and its significance for the estimation of time of death. J Med Entomol 57:1373–1381. https://doi.org/10.1093/jme/tjaa046
Catts PE, Haskell NH (1990) Entomology and death: a procedurals guide, 3rd edn. Joyce’s Print Shop, Clemson, pp 52–97
Speight MR, Hunter MD, Watt AD (2008) Insects and climate. Ecology of insects: concepts and applications. 2nd edn, Wiley-Blackwell, Hoboken, NJ, pp 33–60
Bai Y, Dong JJ, Guan DL et al (2016) Geographic variation in wing size and shape of the grasshopper Trilophidia annulata (Orthoptera: Oedipodidae): morphological trait variations follow an ecogeographical rule. Sci Rep 6:1–15. https://doi.org/10.1038/srep32680
Owings CG, Spiegelman C, Tarone AM et al (2014) Developmental variation among Cochliomyia macellaria Fabricius (Diptera: Calliphoridae) populations from three ecoregions of Texas, USA. Int J Legal Med 128:709–717. https://doi.org/10.1007/s00414-014-1014-0
Valladares F, Matesanz S, Guilhaumon F et al (2014) The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol Lett 17:1351–1364. https://doi.org/10.1111/ele.12348
Grassberger M, Reiter C (2001) Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen-and isomorphen-diagram. Forensic Sci Int 120:32–36
Roe A, Higley LG (2015) Development modeling of Lucilia sericata (Diptera: Calliphoridae). PeerJ 3:e803
Pruna W, Guarderas P, Donoso DA et al (2019) Life cycle of Lucilia sericata (Meigen 1826) collected from Andean mountains. Neotrop Biodivers 5:3–9
Wells JD (1991) Chrysomya megacephala (Diptera: Calliphoridae) has reached the continental United States: review of its biology, pest status, and spread around the world. J Med Entomol 28:471–473. https://doi.org/10.1093/jmedent/28.3.471
Badenhorst R, Villet MH (2018) The uses of Chrysomya megacephala (Fabricius, 1794)(Diptera: Calliphoridae) in forensic entomology. Forensic Sci Res 3:2–15. https://doi.org/10.1080/20961790.2018.1426136
Sukontason KL, Narongchai P, Sripakdee D et al (2005) First report of human myiasis caused by Chrysomya megacephala and Chrysomya rufifacies (Diptera: Calliphoridae) in Thailand, and its implication in forensic entomology. J Med Entomol 42:702–704. https://doi.org/10.1093/jmedent/42.4.702
Olsen AR, Sidebottom TH, Benett SG (1993) The Oriental latrine fly, Chrysomya megacephala (Fabricius 1794)(Diptera: Calliphoridae), as an invading blowfly of public health importance. Bull Soc Vector Ecol 18:133–146
Kurahashi H (1978) The oriental latrine fly: Chrysomya megacephala (Fabricius) newly recorded from Ghana and Senegal, West Africa. Kontyû 46:432
Williams KA, Villet MH (2006) A new and earlier record of Chrysomya megacephala in South Africa, with notes on another exotic species, Calliphora vicina (Diptera: Calliphoridae). Afr Invert 47:347–350
Baumgartner DL, Greenberg B (1984) The genus Chrysomya (Diptera: Calliphoridae) in the new world. J Med Entomol 21:105–113. https://doi.org/10.1093/jmedent/21.1.105
Kurahashi H, Wells JD, Ogino K (1994) The oriental latrine fly, Chrysomya megacephala (Fabricus)(Diptera), newly recorded from Honduras, Central America. Japan J Entomol 62:860
Guimarães J, do Prado A, Linhares AX (1978) Three newly introduced blowfly species in southern Brazil (Diptera, Calliphoridae). Rev Bras Entomol 22:53–60
Barrios BB, Peris SV (1984) Chrysomya megacephala (FABR., 1784) en Paraguay. Eos 59:17
Olsen AR, Angold SC, Gross DF et al (1992) New record of the blowfly, Chrysomya megacephala (Fabr.), from Ecuador (Diptera: Calliphoridae). Pan-Pac Entomol 68:280–281
Schnack JA, Mariluis JC (1995) Status of Chrysomya blow flies (Diptera: Calliphoridae) in Argentina. J Vector Ecol 20:189–194
Yusseff-Vanegas SZ, Agnarsson I (2017) DNA-barcoding of forensically important blow flies (Diptera: Calliphoridae) in the Caribbean Region. PeerJ 5. https://doi.org/10.7717/peerj.3516
Megna YSM, Mayet YL, Abreu YJ (2021) Primer reporte del género Chrysomya Robineau-Desvoidy (Diptera: Calliphoridae) en Cuba: su importancia criminalística. Bol SEA 68:194–198
Pai CY, Jien MC, Li L et al (2007) Application of forensic entomology to postmortem interval determination of a burned human corpse: a homicide case report from southern Taiwan. J Formos Med Assoc 106:792–798. https://doi.org/10.1016/S0929-6646(08)60043-1
Thevan K, Ahmad AH, Md Rawi CS et al (2010) Growth of Chrysomya megacephala (Fabricius) maggots in a morgue cooler. J Forensic Sci 55:1656–1658. https://doi.org/10.1111/j.1556-4029.2010.01485.x
Yang YQ, Li XB, Shao RY et al (2016) Developmental times of Chrysomya megacephala (Fabricius)(Diptera: Calliphoridae) at constant temperatures and applications in forensic entomology. J Forensic Sci 61:1278–1284. https://doi.org/10.1111/1556-4029.13159
Whitworth T (2006) Keys to the genera and species of blow flies (Diptera: Calliphoridae) of America, North of Mexico. Proc Entomol Soc Wash 108:689–725
Schneider C, Rasband W, Eliceiri K (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Rodríguez-Estrella R (2007) Land use changes affect distributional patterns of desert birds in the Baja California peninsula, México. Divers Distrib 13:877–889. https://doi.org/10.1111/j.1472-4642.2007.00387.x
Jácome-Flores ME, Blazquez MC, Sosa VJ et al (2015) Type of soil and temperature range explain the preferred habitat and current distribution of the endemic lizard Aspidoscelis hyperythra in southern Baja California peninsula. J Arid Environ 113:126–133
Tellería JL, Fernández-López J, Fandos G (2016) Effect of climate change on mediterranean winter ranges of two migratory passerines. PLoS ONE 11:e0146958. https://doi.org/10.1371/journal.pone.0146958
Rcore Team (2020) R: a language and environment for statistical computing: Vienna, Austria, R Foundation for Statistical Computing, http://www.R-project.org
Wilson L, Barnett W (1983) Degree-days: an aid in crop and pest management. Calif Agric 37:4–7
Ames C, Turner B (2003) Low temperature episodes in development of blowflies: implications for postmortem interval estimation. Med Vet Entomol 17:178–186. https://doi.org/10.1046/j.1365-2915.2003.00421.x
Subramanian H, Mohan KR (1980) Biology of the blowflies Chrysomyia megacephala, Chrysomyia rufifacies and Lucilia cuprina. Kerala J Vet Sci 11:252–261
O’Flynn MA (1983) The succession and rate of development of blowflies in carrion in southern Queensland and the application of these data to forensic entomology. J Aust Entomol Soc 22:137–147. https://doi.org/10.1111/j.1440-6055.1983.tb01860.x
Wells JD, Kurahashi H (1994) Chrysomya megacephala (Fabricius)(Diptera: Calliphoridae) development: rate, variation and the implications for forensic entomology. Med Entomol Zool 45:303–309. https://doi.org/10.7601/mez.45.303_1
Gabre RM, Adham FK, Chi H (2005) Life table of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae). Acta Oecol 27:179–183. https://doi.org/10.1016/j.actao.2004.12.002
Bharti M, Singh D, Sharma YP (2007) Effect of temperature on the development of forensically important blowfly, Chrysomya megacephala (Fabricius)(Diptera: Calliphoridae). Entomon 32:149–151
Rabêlo KCN, Thyssen P, Salgado RL et al (2011) Bionomics of two forensically important blowfly species Chrysomya megacephala and Chrysomya putoria (Diptera: Calliphoridae) reared on four types of diet. Forensic Sci Int 210:257–262. https://doi.org/10.1016/j.forsciint.2011.03.022
Arias-Di Donato L, Liria J (2016) Vital statistics of Chrysomya megacephala (Fabricius, 1794)(Diptera: Calliphoridae) under different diets from Venezuela. J Entomol Zool Stud 4:247–251
Abd Algalil FM, Zambare SP (2015) Effects of temperature on the development of Calliphorid fly of forensic importance Chrysomya megacephala (Fabricius, 1794). Indian J Appl Res 5:767–769
Gruner SV, Slone DH, Capinera JL et al (2017) Development of the Oriental latrine fly, Chrysomya megacephala (Diptera: Calliphoridae), at five constant temperatures. J Med Entomol 54:290–298. https://doi.org/10.1093/jme/tjw169
Zhang Y, Wang Y, Yang L et al (2018) Development of Chrysomya megacephala at constant temperatures within its colony range in Yangtze River Delta region of China. Forensic Sci Res 3:74–82. https://doi.org/10.1080/20961790.2017.1403007
Bambaradeniya YTB, Karunaratne I, Tomberlin JK et al (2019) Effect of temperature and tissue type on the development of the forensic fly Chrysomya megacephala (Diptera: Calliphoridae). J Med Entomol 56:1571–1581. https://doi.org/10.1093/jme/tjz097
Salleh AFM, Talib A, Marwi MA et al (2009) Effects of temperatures on larval development of Chrysomya megacephala (Fabricius) and Chrysomya rufifacies (Macquart)(Diptera: Calliphoridae): application in forensic science. Malay J Health Sci 7:89–96
Ismail MI, Osman K, King O et al (2007) Accelerating Chrysomya megacephala maggot growth for forensic entomology cases. Jurnal Sains Kesihatan Malays 5:17–26
Niederegger S, Pastuschek J, Mall G (2010) Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci Int 199:72–78. https://doi.org/10.1016/j.forsciint.2010.03.015
Dadour IR, Cook DF, Fissioli JN et al (2010) Forensic entomology: application, education and research in Western Australia. Forensic Sci Int 120:48–52. https://doi.org/10.1016/S0379-0738(01)00420-0
Tarone AM, Foran DR (2008) Generalized additive models and Lucilia sericata growth: assessing confidence intervals and error rates in forensic entomology. J Forensic Sci 53:942–948. https://doi.org/10.1111/j.1556-4029.2008.00744.x
Anderson GS (2000) Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J Forensic Sci 45:824–832. https://doi.org/10.1520/JFS14778J
Núñez-Váquez C, Tomberlin JK, Cantú-Sifuentes M et al (2013) Laboratory development and field validation of Phormia regina (Diptera: Calliphoridae). J Med Entomol 50:252–260. https://doi.org/10.1603/ME12114
Yang YQ, Lyu Z, Li XB et al (2015) Development of Hemipyrellia ligurriens (Wiedemann) (Diptera: Calliphoridae) at constant temperatures: applications in estimating postmortem interval. Forensic Sci Int 253:48–54. https://doi.org/10.1016/j.forsciint.2015.05.006
Wang J, Hu Z, Chen Y et al (2002) Effects of temperature on the larval body length changes of Chrysomya megacepala (Fabricius). Acta Parasitol Med Entomol Sin 9:100–105
Sukontason K, Piangjai S, Siriwattanarungsee S et al (2008) Morphology and developmental rate of blowflies Chrysomya megacephala and Chrysomya rufifacies in Thailand: application in forensic entomology. Parasitol Res 102:1207–1216. https://doi.org/10.1007/s00436-008-0895-6
Armbruster P, Conn JE (2006) Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann Entomol Soc Am 99:1234–1243. https://doi.org/10.1603/0013-8746(2006)99[1234:GVOLGI]2.0.CO;2
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Blanckenhorn WU, Whitman DW, Ananthakrishnan TN (2009) Causes and consequences of phenotypic plasticity in body size: the case of the yellow dung fly Scathophaga stercoraria (Diptera: Scathophagidae). In: Whitman DW, Ananthakrishnan TN. Phenotypic plasticity of insects: mechanisms and consequences. Science Publishers, Enfield, NH, pp 369–422, NH: CRC Press
Tarone AM (2007) Lucilia sericata development: plasticity, population differences and gene expression. East Lansing: Michigan State University. Thesis Dissertation pp 248
Gallagher MB, Sandhu S, Kimsey R (2010) Variation in developmental time for geographically distinct populations of the common green bottle fly, Lucilia sericata (Meigen). J Forensic Sci 55:438–442. https://doi.org/10.1111/j.1556-4029.2009.01285.x
Richards CS, Paterson ID, Villet MH (2008) Estimating the age of immature Chrysomya albiceps (Diptera: Calliphoridae), correcting for temperature and geographical latitude. Int J Leg Med 122:271–279. https://doi.org/10.1007/s00414-007-0201-7
Trudgill DL, Perry JN (1994) Thermal time and ecological strategies-a unifying hypothesis. Ann Appl Biol 125:521–532. https://doi.org/10.1111/j.1744-7348.1994.tb04989.x
Trudgill DL (1995) Why do tropical poikilothermic organisms tend to have higher threshold temperature for development than temperate ones. Funct Ecol 9:136–137
Stillwell RC, Fox CW (2005) Complex patterns of phenotypic plasticity: interactive effects of temperature during rearing and oviposition. Ecology 86:924–934
Greenberg B, Kunich JC (2002) Entomology and the law: flies as forensic indicators. Cambridge Univ Press, Cambridge, UK
Norry FM, Bubliy OA, Loeschcke V (2001) Developmental time, body size and wing loading in Drosophila buzzatii from lowland and highland populations in Argentina. Hereditas 135:35–40
Conner JK, Hartl DL (2004) A primer of ecological genetics. Sinauer Inc, Sunderland, MA
Tarone AM, Picard CJ, Spiegelman et al (2011) Population and temperature effects on Lucilia sericata (Diptera: Calliphoridae) body size and minimum development time. J Med Entomol 48:1062-1068
Hu Y, Yuan X, Zhu F et al (2010) Development time and size-related traits in the oriental blowfly, Chrysomya megacephala along a latitudinal gradient from China. J Therm Biol 35:366–371
Salem AM, Adham FK, Picard CJ (2015) Survey of the genetic diversity of forensically important Chrysomya (Diptera: Calliphoridae) from Egypt. J Med Entomol 52:320–328. https://doi.org/10.1093/jme/tjv013
Chong YV, Chua TH, Song BK (2014) Genetic variations of Chrysomya megacephala populations in Malaysia (Diptera: Calliphoridae). Adv Entomol 2:49–56. https://doi.org/10.4236/ae.2014.21009
Barton M, Sunnucks P, Norgate M et al (2014) Co-gradient variation in growth rate and development time of a broadly distributed butterfly. PLoS ONE 9:e95258. https://doi.org/10.1371/journal.pone.0095258
Cronin AL, Schwarz MP (1999) Latitudinal variation in the life cycle of allodapine bees (Hymenoptera; Apidae). Can J Zool 77:857–864. https://doi.org/10.1139/cjz-77-6-857
Tang J, He H, Chen C et al (2017) Latitudinal cogradient variation of development time and growth rate and a negative latitudinal body weight cline in a widely distributed cabbage beetle. PLoS ONE 12:e0181030. https://doi.org/10.1371/journal.pone.0181030
Khan HAA, Khan MU, Nasiba A (2019) Geographical variations in life histories of house flies, Musca domestica (Diptera: Muscidae), in Punjab, Pakistan. J Med Entomol 56:1225–1230. https://doi.org/10.1093/jme/tjz069
Jaworski T, Hilszczański J (2013) The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the context of expected climate change. For Res Pap 74:345–355. https://doi.org/10.2478/frp-2013-0033
Wigglesworth VB (2012) The principles of insect physiology. Cambridge University Press, New York, NY
Fatchurochim S, Geden CJ, Axtell RC (1989) Filth fly (Diptera) oviposition and larval development in poultry manure of various moisture levels. J Entomol Sci 24:224–231. https://doi.org/10.18474/0749-8004-24.2.224
Nielsen BO, Nielsen SA (1976) Schmeissfliegen (Calliphoridae) und vakuumverpackter Schinken. Anz Schädlingskd Pfl Umwelt 49:113–115. https://doi.org/10.1007/BF01985066
Acknowledgements
The authors thank the Centro Regional de Investigación en Salud Pública of the Instituto Nacional de Salud Pública (CRISP/INSP) of Tapachula, Chiapas, Mexico, for providing their facilities for the development of this research. The main author, Ana Julia Pereira from Argentina, would like to thank the Mexican government for its support through the program “Becas de Excelencia para Extranjeros” to carry out a short research stay in Mexico, where this research was developed under the direction of Dr. Nuñez.
Funding
This study was partially funded by the “Secretaría de Relaciones Exteriores” from Mexico in the program “Becas de Excelencia para Extranjeros” call 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, A.J., Centeno, N.D. & Nuñez-Vázquez, C. Effects of population variations and temperature on Chrysomya megacephala (Diptera: Calliphoridae) development: implications for estimating the postmortem interval. Int J Legal Med 138, 165–175 (2024). https://doi.org/10.1007/s00414-023-03020-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-023-03020-2