Abstract
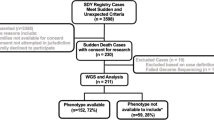
Cardiac diseases and sudden cardiac death (SCD) are more prevalent in individuals diagnosed with schizophrenia compared to the general population, with especially coronary artery disease (CAD) as the major cardiovascular cause of death. Antipsychotic medications, genetics, and lifestyle factors may contribute to the increased SCD in individuals with schizophrenia. The role of antipsychotic medications and lifestyle factors have been widely investigated, while the genetic predisposition to inherited cardiac diseases in schizophrenia is poorly understood. In this study, we examined 100 genes associated with inherited cardiomyopathies and cardiac channelopathies in 97 deceased individuals diagnosed with schizophrenia for the prevalence of genetic variants associated with SCD. The deceased individuals had various causes of death and were included in the SURVIVE project, a prospective, autopsy-based study of mentally ill individuals in Denmark. This is the first study of multiple inherited cardiac disease-related genes in deceased individuals with diagnosed schizophrenia to shed light on the genetic predisposition to SCD in individuals with schizophrenia. We found no evidence for an overrepresentation of rare variants with high penetrance in inherited cardiac diseases, following the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG) consensus guidelines. However, we found that the deceased individuals had a statistically significantly increased polygenic burden caused by variants in the investigated heart genes compared to the general population. This indicates that common variants with smaller effects in heart genes may play a role in schizophrenia.

Similar content being viewed by others
References
McGrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76. https://doi.org/10.1093/epirev/mxn001
Nordentoft M, Wahlbeck K, Hallgren J et al (2013) Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark. Finland and Sweden PLoS One 8:e55176. https://doi.org/10.1371/journal.pone.0055176
Westman J, Eriksson SV, Gissler M et al (2018) Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiol Psychiatr Sci 27:519–527. https://doi.org/10.1017/S2045796017000166
Laursen TM (2011) Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res 131:101–104. https://doi.org/10.1016/j.schres.2011.06.008
Kritharides L, Chow V, Lambert TJ (2017) Cardiovascular disease in patients with schizophrenia. Med J Aust 206:91–95. https://doi.org/10.5694/mja16.00650
Laursen TM, Nordentoft M (2011) Heart disease treatment and mortality in schizophrenia and bipolar disorder-changes in the Danish population between 1994 and 2006. J Psychiatr Res 45:29–35. https://doi.org/10.1016/j.jpsychires.2010.04.027
Laursen TM, Munk-Olsen T, Agerbo E et al (2009) Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry 66:713–720. https://doi.org/10.1001/archgenpsychiatry.2009.61
Heiberg IH, Jacobsen BK, Balteskard L et al (2019) Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatr Scand 139:558–571. https://doi.org/10.1111/acps.13017
Laursen TM, Nordentoft M, Mortensen PB (2014) Excess early mortality in schizophrenia. Annu Rev Clin Psychol 10:425–448. https://doi.org/10.1146/annurev-clinpsy-032813-153657
Laursen TM, Munk-Olsen T, Vestergaard M (2012) Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry 25:83–88. https://doi.org/10.1097/YCO.0b013e32835035ca
Risgaard B, Waagstein K, Winkel BG et al (2015) Sudden cardiac death in young adults with previous hospital-based psychiatric inpatient and outpatient treatment: a nationwide cohort study from Denmark. J Clin Psychiatry 76:e1122–e1129. https://doi.org/10.4088/JCP.14m09742
Jindal R, MacKenzie EM, Baker GB, Yeragani VK (2005) Cardiac risk and schizophrenia. J Psychiatry Neurosci 30(6):393–395
Blom MT, Cohen D, Seldenrijk A et al (2014) Brugada syndrome ECG is highly prevalent in schizophrenia. Circ Arrhythm Electrophysiol 7:384–391. https://doi.org/10.1161/CIRCEP.113.000927
Sutterland AL, Blom MT, Ladee K et al (2019) Increased prevalence of ECG suspicious for Brugada Syndrome in recent onset schizophrenia spectrum disorders. Schizophr Res 210:59–65. https://doi.org/10.1016/j.schres.2019.06.013
Huertas-Vazquez A, Teodorescu C, Reinier K et al (2013) A common missense variant in the neuregulin 1 gene is associated with both schizophrenia and sudden cardiac death. Hear Rhythm 10:994–998. https://doi.org/10.1016/j.hrthm.2013.03.020
Chugh SS, Senashova O, Watts A et al (2004) Postmortem molecular screening in unexplained sudden death. J Am CollCardiol 43:1625–1629. https://doi.org/10.1016/j.jacc.2003.11.052
Skinner JR, Crawford J, Smith W et al (2011) Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Hear Rhythm 8:412–419. https://doi.org/10.1016/j.hrthm.2010.11.016
Alcalde M, Campuzano O, Allegue C et al (2015) SequenomMassARRAY approach in the arrhythmogenic right ventricular cardiomyopathy post-mortem setting: clinical and forensic implications. Int J Leg Med 129:1–10. https://doi.org/10.1007/s00414-014-0996-y
Zhang M, Tavora F, Oliveira JB et al (2012) PKP2 mutations in sudden death from arrhythmogenic right ventricular cardiomyopathy (ARVC) and sudden unexpected death with negative autopsy (SUDNA). Circ J 76:189–194
Allegue C, Gil R, Blanco-Verea A et al (2011) Prevalence of HCM and long QT syndrome mutations in young sudden cardiac death-related cases. Int J Leg Med 125:565–572. https://doi.org/10.1007/s00414-011-0572-7
Hertz CL, Christiansen SL, Ferrero-Miliani L et al (2016) Next-generation sequencing of 100 candidate genes in young victims of suspected sudden cardiac death with structural abnormalities of the heart. Int J Leg Med 130:91–102. https://doi.org/10.1007/s00414-015-1261-8
Neubauer J, Haas C, Bartsch C et al (2016) Post-mortem whole-exome sequencing (WES) with a focus on cardiac disease-associated genes in five young sudden unexplained death (SUD) cases. Int J Leg Med 130:1011–1021. https://doi.org/10.1007/s00414-016-1317-4
Nunn LM, Lopes LR, Syrris P et al (2016) Diagnostic yield of molecular autopsy in patients with sudden arrhythmic death syndrome using targeted exome sequencing. Europace 18:888–896. https://doi.org/10.1093/europace/euv285
Bagnall RD, Weintraub RG, Ingles J et al (2016) A prospective study of sudden cardiac death among children and young adults. N Engl J Med 374:2441–2452. https://doi.org/10.1056/NEJMoa1510687
Bagnall RD, Das KJ, Duflou J, Semsarian C (2014) Exome analysis-based molecular autopsy in cases of sudden unexplained death in the young. Hear Rhythm 11:655–662. https://doi.org/10.1016/j.hrthm.2014.01.017
Narula N, Tester DJ, Paulmichl A et al (2015) Post-mortem whole exome sequencing with gene-specific analysis for autopsy-negative sudden unexplained death in the young: a case series. PediatrCardiol 36:768–778. https://doi.org/10.1007/s00246-014-1082-4
Christiansen SL, Hertz CL, Ferrero-Miliani L et al (2016) Genetic investigation of 100 heart genes in sudden unexplained death victims in a forensic setting. Eur J Hum Genet 24:1797–1802. https://doi.org/10.1038/ejhg.2016.118
Tester DJ, Medeiros-Domingo A, Will ML et al (2012) Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc 87:524–539. https://doi.org/10.1016/j.mayocp.2012.02.017
Hata Y, Kinoshita K, Mizumaki K et al (2016) Postmortem genetic analysis of sudden unexplained death syndrome under 50 years of age: a next-generation sequencing study. Hear Rhythm 13:1544–1551. https://doi.org/10.1016/j.hrthm.2016.03.038
Lahrouchi N, Raju H, Lodder EM et al (2017) Utility of post-mortem genetic testing in cases of sudden arrhythmic death syndrome. J Am CollCardiol 69:2134–2145. https://doi.org/10.1016/j.jacc.2017.02.046
Shanks GW, Tester DJ, Nishtala S, et al (2017) Genomic triangulation and coverage analysis in whole-exome sequencing-based molecular autopsies. Circ Cardiovasc Genet 10:e001828. https://doi.org/10.1161/CIRCGENETICS.117.001828
Hellenthal N, Gaertner-Rommel A, Klauke B et al (2017) Molecular autopsy of sudden unexplained deaths reveals genetic predispositions for cardiac diseases among young forensic cases. Europace 19:1881–1890. https://doi.org/10.1093/europace/euw247
Neubauer J, Lecca MR, Russo G et al (2018) Exome analysis in 34 sudden unexplained death (SUD) victims mainly identified variants in channelopathy-associated genes. Int J Leg Med 132:1057–1065. https://doi.org/10.1007/s00414-018-1775-y
Hertz CL, Christiansen SL, Larsen MK et al (2016) Genetic investigations of sudden unexpected deaths in infancy using next-generation sequencing of 100 genes associated with cardiac diseases. Eur J Hum Genet 24:817–822. https://doi.org/10.1038/ejhg.2015.198
Kauferstein S, Kiehne N, Jenewein T et al (2013) Genetic analysis of sudden unexplained death: a multidisciplinary approach. Forensic Sci Int 229:122–127. https://doi.org/10.1016/j.forsciint.2013.03.050
Hertz CL, Christiansen SL, Ferrero-Miliani L et al (2015) Next-generation sequencing of 34 genes in sudden unexplained death victims in forensics and in patients with channelopathic cardiac diseases. Int J Leg Med 129:793–800. https://doi.org/10.1007/s00414-014-1105-y
Winkel BG, Larsen MK, Berge KE et al (2012) The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol 23:1092–1098. https://doi.org/10.1111/j.1540-8167.2012.02371.x
Larsen MK, Christiansen SL, Hertz CL et al (2020) Targeted molecular genetic testing in young sudden cardiac death victims from Western Denmark. Int J Leg Med 134:111–121. https://doi.org/10.1007/s00414-019-02179-x
Larsen MK, Berge KE, Leren TP et al (2013) Postmortem genetic testing of the ryanodine receptor 2 (RYR2) gene in a cohort of sudden unexplained death cases. Int J Leg Med 127:139–144. https://doi.org/10.1007/s00414-011-0658-2
Larsen MK, Nissen PH, Berge KE et al (2012) Molecular autopsy in young sudden cardiac death victims with suspected cardiomyopathy. Forensic Sci Int 219:33–38. https://doi.org/10.1016/j.forsciint.2011.11.020
Cann F, Corbett M, O’Sullivan D et al (2017) Phenotype-driven molecular autopsy for sudden cardiac death. Clin Genet 91:22–29. https://doi.org/10.1111/cge.12778
Banner J, Hoyer CB, Christensen MR et al (2018) SURVIVE: let the dead help the living-an autopsy-based cohort study for mapping risk markers of death among those with severe mental illnesses. Scand J Forensic Sci 24:7–17. https://doi.org/10.2478/sjfs-2018-0002
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Karbassi I, Maston GA, Love A et al (2016) A standardized DNA variant scoring system for pathogenicity assessments in Mendelian disorders. Hum Mutat 37:127–134. https://doi.org/10.1002/humu.22918
Andersen JD, Jacobsen SB, Trudsø LC et al (2019). Whole genome and transcriptome sequencing of post-mortem cardiac tissues from sudden cardiac death victims identifies a gene regulatory variant in NEXN. https://doi.org/10.1007/s00414-019-02127-9
Grantham R (1974) Amino acid difference formula to help explain protein evolution. Science 185(4154):862–864. https://doi.org/10.1126/science.185.4154.862
Mathe E, Olivier M, Kato S et al (2006) Computational approaches for predicting the biological effect of p53 missense mutations: a comparison of three sequence analysis based methods. Nucleic Acids Res 34:1317–1325. https://doi.org/10.1093/nar/gkj518
Henikoff S, Henikoff JG (1992) Amino acid substitution matrices from protein blocks. Proc Natl AcadSci U S A 89:10915–10919
Tavtigian SV, Oefner PJ, Babikyan D et al (2009) Rare, evolutionarily unlikely missense substitutions in ATM confer increased risk of breast cancer. Am J Hum Genet 85(4):427–446. https://doi.org/10.1016/j.ajhg.2009.08.018
Stone EA, Sidow A (2005) Physicochemical constraint violation by missense substitutions mediates impairment of protein function and disease severity. Genome Res 15:978–986. https://doi.org/10.1101/gr.3804205
Ng PC, Henikoff S (2001) Predicting deleterious amino acid substitutions. Genome Res 11:863–874. https://doi.org/10.1101/gr.176601
Schwarz JM, Rodelsperger C, Schuelke M, Seelow D (2010) MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7:575–576. https://doi.org/10.1038/nmeth0810-575
Sherry ST, Ward MH, Kholodov M et al (2001) dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 29:308–311
Karczewski KJ, Weisburd B, Thomas B et al (2017) The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res 45:D840–D845. https://doi.org/10.1093/nar/gkw971
Stenson PD, Ball EV, Mort M et al (2003) Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21:577–581. https://doi.org/10.1002/humu.10212
Landrum MJ, Lee JM, Riley GR et al (2014) ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 42:D980–D985. https://doi.org/10.1093/nar/gkt1113
Ng D, Johnston JJ, Teer JK et al (2013) Interpreting secondary cardiac disease variants in an exome cohort. CircCardiovasc Genet 6:337–346. https://doi.org/10.1161/CIRCGENETICS.113.000039
Lahrouchi N, Raju H, Lodder EM et al (2020) The yield of postmortem genetic testing in sudden death cases with structural findings at autopsy. Eur J Hum Genet 28:17–22. https://doi.org/10.1038/s41431-019-0500-8
Rentzsch P, Witten D, Cooper GM et al (2019) CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. https://doi.org/10.1093/nar/gky1016
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Karczewski KJ, Francioli LC, Tiao G et al (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. https://doi.org/10.1038/s41586-020-2308-7
Imbrici P, Camerino DC, Tricarico D (2013) Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. https://doi.org/10.3389/fgene.2013.00076
Espregueira Themudo G, Leerschool AR, Rodriguez-Proano C et al (2020) Targeted exon sequencing in deceased schizophrenia patients in Denmark. Int J Leg Med 134:135–147. https://doi.org/10.1007/s00414-019-02212-z
Buch AK, Busch J, Ylijoki-Sørensen S, Banner J (2018) Factors associated with autopsy rates in a 6-year sample of Danish suicides in the Capital area of Copenhagen. J Forensic Leg Med. https://doi.org/10.1016/j.jflm.2018.09.009
Risgaard B, Jabbari R, Refsgaard L et al (2013) High prevalence of genetic variants previously associated with Brugada syndrome in new exome data. Clin Genet 84:489–495. https://doi.org/10.1111/cge.12126
Paludan-Muller C, Ahlberg G, Ghouse J et al (2017) Integration of 60,000 exomes and ACMG guidelines question the role of Catecholaminergic Polymorphic Ventricular Tachycardia-associated variants. Clin Genet 91:63–72. https://doi.org/10.1111/cge.12847
Nouhravesh N, Ahlberg G, Ghouse J et al (2016) Analyses of more than 60,000 exomes questions the role of numerous genes previously associated with dilated cardiomyopathy. Mol Genet Genomic Med 4:617–623. https://doi.org/10.1002/mgg3.245
Watkins H, McKenna WJ, Thierfelder L et al (1995) Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med 332:1058–1064. https://doi.org/10.1056/NEJM199504203321603
Gimeno JR, Monserrat L, Perez-Sanchez I et al (2009) Hypertrophic cardiomyopathy. A study of the troponin-T gene in 127 Spanish families. Rev EspCardiol 62:1473–1477
Garcia-Castro M, Reguero JR, Batalla A et al (2003) Hypertrophic cardiomyopathy: low frequency of mutations in the beta-myosin heavy chain (MYH7) and cardiac troponin T (TNNT2) genes among Spanish patients. Clin Chem 49:1279–1285. https://doi.org/10.1373/49.8.1279
Millat G, Bouvagnet P, Chevalier P et al (2011) Clinical and mutational spectrum in a cohort of 105 unrelated patients with dilated cardiomyopathy. Eur J Med Genet 54:e570–e575. https://doi.org/10.1016/j.ejmg.2011.07.005
Ripoll-Vera T, Gamez JM, Govea N et al (2016) Clinical and prognostic profiles of cardiomyopathies caused by mutations in the troponin T gene. Rev Esp Cardiol (Engl Ed) 69:149–158. https://doi.org/10.1016/j.rec.2015.06.025
Perez-Pinar M, Mathur R, Foguet Q et al (2016) Cardiovascular risk factors among patients with schizophrenia, bipolar, depressive, anxiety, and personality disorders. Eur Psychiatry 35:8–15. https://doi.org/10.1016/j.eurpsy.2016.02.004
Vancampfort D, Firth J, Schuch FB et al (2017) Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry 16:308–315. https://doi.org/10.1002/wps.20458
Straus SMJM, Sturkenboom MCJM, Bleumink GS et al (2005) Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. https://doi.org/10.1093/eurheartj/ehi312
Simpson TF, Salazar JW, Vittinghoff E et al (2020) Association of QT-prolonging medications with risk of autopsy-defined causes of sudden death. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.0148
Fahed AC, Wang M, Homburger JR et al (2020) Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat Commun. https://doi.org/10.1038/s41467-020-17374-3
Guipponi M, Santoni FA, Setola V et al (2014) Exome sequencing in 53 sporadic cases of schizophrenia identifies 18 putative candidate genes. PLoS ONE 9:e112745. https://doi.org/10.1371/journal.pone.0112745
Girard SL, Gauthier J, Noreau A et al (2011) Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet 43:860–863. https://doi.org/10.1038/ng.886
Schizophrenia Working Group of the Psychiatric Genomics C (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. https://doi.org/10.1038/nature13595
Bezzina CR, Barc J, Mizusawa Y et al (2013) Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat Genet 45:1044–1049. https://doi.org/10.1038/ng.2712
Lahrouchi N, Tadros R, Crotti L et al (2020) Transethnic genome-wide association study provides insights in the genetic architecture and heritability of long QT syndrome. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.045956
Purcell SM, Wray NR, Stone JL et al (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. https://doi.org/10.1038/nature08185
Vasilescu C, Ojala TH, Brilhante V et al (2018) Genetic basis of severe childhood-onset cardiomyopathies. J Am Coll Cardiol 72:2324–2338. https://doi.org/10.1016/j.jacc.2018.08.2171
Mohler PJ, Schott JJ, Gramolini AO et al (2003) Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421:634–639. https://doi.org/10.1038/nature01335
Moss AJ, Shimizu W, Wilde AA et al (2007) Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation 115:2481–2489. https://doi.org/10.1161/CIRCULATIONAHA.106.665406
Kapplinger JD, Tester DJ, Alders M et al (2010) An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Hear Rhythm 7:33–46. https://doi.org/10.1016/j.hrthm.2009.09.069
Hofman-Bang J, Behr ER, Hedley P et al (2006) High-efficiency multiplex capillary electrophoresis single strand conformation polymorphism (multi-CE-SSCP) mutation screening of SCN5A: a rapid genetic approach to cardiac arrhythmia. Clin Genet 69:504–511. https://doi.org/10.1111/j.1399-0004.2006.00621.x
Acknowledgements
We would like to thank the next-of-kin of study participants for providing consent for inclusion in this study, Anja Ladegaard Jørgensen for technical assistance in the laboratory, and the people involved in the SURVIVE project, including medical doctors, secretaries, and forensic and laboratory technicians at the forensic medicine departments in Odense, Aarhus, and Copenhagen, Denmark.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics
The experiments were performed in accordance with relevant guidelines and regulations, and the study was approved by the Danish National Research Ethics Board (journal no. 1305373) and the National Danish Data Protection Agency (SUND-2016–06).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Christiansen, S.L., Andersen, J.D., Themudo, G.E. et al. Genetic investigations of 100 inherited cardiac disease-related genes in deceased individuals with schizophrenia. Int J Legal Med 135, 1395–1405 (2021). https://doi.org/10.1007/s00414-021-02595-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-021-02595-y