Abstract
The identification of mixed stains has always been a difficult problem in personal identification in the forensic field. In recent years, tissue-specific methylation sites have proven to be very stable biomarkers for distinguishing tissue origin. However, it is still challenging to perform tissue source identification and individual identification simultaneously. In this study, we developed a method that uses tissue-specific methylation markers combined with single-nucleotide polymorphism (SNP) markers to detect semen from mixed biofluids and to identify individuals simultaneously. Semen-specific CpG markers were chosen from the literature and further validated utilizing methylation-sensitive restriction endonuclease (MSRE) combined with PCR technology. The neighboring SNP markers were searched in the flanking sequence of the target CpG within 400 bp, and SNP typing was then carried out through a single-base extension reaction followed by capillary electrophoresis. Eventually, a method of MSRE combined with SNaPshot that could detect 12 compound CpG-SNP markers was developed. Using this system, 10 ng of total DNA and DNA mixture with semen content up to 25% could be typed successfully. Moreover, the cumulative discrimination power of the system in the northern Chinese Han population is 0.9998. This study provides a valuable strategy for forensic practice to perform tissue origin and individual identification from mixed stains simultaneously.





Similar content being viewed by others
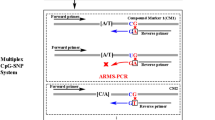
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary materials.
Code availability
Not applicable.
References
Gill P, Jeffreys AJ, Werrett DJ (1985) Forensic application of DNA ‘fingerprints.’ Nature 318(6046):577–579. https://doi.org/10.1038/318577a0
Li CX, Han JP, Ren WY, Ji AQ, Xu XL, Hu L (2011) DNA profiling of spermatozoa by laser capture microdissection and low volume-PCR. PLoS ONE 6(8):e22316. https://doi.org/10.1371/journal.pone.0022316
Naue J, Hoefsloot HCJ, Kloosterman AD, Verschure PJ (2018) Forensic DNA methylation profiling from minimal traces: how low can we go? Forensic SciInt Genet 33:17–23. https://doi.org/10.1016/j.fsigen.2017.11.004
Kayser M (2017) Forensic use of Y-chromosome DNA: a general overview. Hum Genet 136(5):621–635. https://doi.org/10.1007/s00439-017-1776-9
Castella V, Gervaix J, Hall D (2013) DIP-STR: highly sensitive markers for the analysis of unbalanced genomic mixtures. Hum Mutat 34(4):644–654. https://doi.org/10.1002/humu.22280
Liu J, Li W, Wang J, Chen D, Liu Z, Shi J, Cheng F, Li Z, Ren J, Zhang G, Yun K (2019) A new set of DIP-SNP markers for detection of unbalanced and degraded DNA mixtures. Electrophoresis 40(14):1795–1804. https://doi.org/10.1002/elps.201900017
Liu J, Hao T, Cheng X, Wang J, Li W, Liu Z, Shi J, Li Z, Ren J, Yun K, Zhang G (2020) DIP-microhaplotypes: new markers for detection of unbalanced DNA mixtures. Int J Legal Med. https://doi.org/10.1007/s00414-020-02288-y
Kelly H, Bright JA, Buckleton JS, Curran JM (2014) A comparison of statistical models for the analysis of complex forensic DNA profiles. Sci Justice 54(1):66–70. https://doi.org/10.1016/j.scijus.2013.07.003
Schaukowitch K, Kim TK (2014) Emerging epigenetic mechanisms of long non-coding RNAs. Neuroscience 264:25–38. https://doi.org/10.1016/j.neuroscience.2013.12.009
Lokk K, Modhukur V, Rajashekar B, Märtens K, Mägi R, Kolde R, Koltšina M, Nilsson TK, Vilo J, Salumets A, Tõnisson N (2014) DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol 15(4):r54. https://doi.org/10.1186/gb-2014-15-4-r54
Silva D, Antunes J, Balamurugan K, Duncan G, Alho CS, McCord B (2016) Developmental validation studies of epigenetic DNA methylation markers for the detection of blood, semen and saliva samples. Forensic SciInt Genet 23:55–63. https://doi.org/10.1016/j.fsigen.2016.01.017
Holtkötter H, Beyer V, Schwender K, Glaub A, Johann KS, Schürenkamp M, Sibbing U, Banken S, Wiegand P, Pfeiffer H, Dennany L, Vennemann M, Vennemann M (2017) Independent validation of body fluid-specific CpG markers and construction of a robust multiplex assay. Forensic SciInt Genet 29:261–268. https://doi.org/10.1016/j.fsigen.2017.05.002
Lin YC, Tsai LC, Lee JC, Liu KL, Tzen JT, Linacre A, Hsieh HM (2016) Novel identification of biofluids using a multiplex methylation-specific PCR combined with single-base extension system. Forensic Sci Med Pathol 12(2):128–138. https://doi.org/10.1007/s12024-016-9763-3
Forat S, Huettel B, Reinhardt R, Fimmers R, Haidl G, Denschlag D, Olek K (2016) Methylation markers for the identification of body fluids and tissues from forensic trace evidence. PLoS ONE 11(2):e0147973. https://doi.org/10.1371/journal.pone.0147973
Zuo T, Tycko B, Liu TM, Lin JJ, Huang TH (2009) Methods in DNA methylation profiling. Epigenomics 1(2):331–345. https://doi.org/10.2217/epi.09.31
Melnikov AA, Gartenhaus RB, Levenson AS, Motchoulskaia NA, LevensonChernokhvostov VV (2005) MSRE-PCR for analysis of gene-specific DNA methylation. Nucleic Acids Res 33(10):e93. https://doi.org/10.1093/nar/gni092
Cheow LF, Quake SR, Burkholder WF, Messerschmidt DM (2015) Multiplexed locus-specific analysis of DNA methylation in single cells. Nat Protoc 10(4):619–631. https://doi.org/10.1038/nprot.2015.041
Lin YC, Tsai LC, Lee JC, Su CW, Tzen JT, Linacre A, Hsieh HM (2016) Novel identification of biofluids using a multiplex methylation sensitive restriction enzyme-PCR system. Forensic SciInt Genet 25:157–165. https://doi.org/10.1016/j.fsigen.2016.08.011
Frumkin D, Wasserstrom A, Budowle B, Davidson A (2011) DNA methylation-based forensic tissue identification. Forensic SciInt Genet 5(5):517–524. https://doi.org/10.1016/j.fsigen.2010.12.001
Wasserstrom A, Frumkin D, Davidson A, Shpitzen M, Herman Y, Gafny R (2013) Demonstration of DSI-semen–A novel DNA methylation-based forensic semen identification assay. Forensic SciInt Genet 7(1):136–142. https://doi.org/10.1016/j.fsigen.2012.08.009
Park J-L, Kwon O-H, Kim JH, Yoo H-S, Lee H-C, Woo K-M, Kim S-Y, Lee S-H, Kim YS (2014) Identification of body fluid-specific DNA methylation markers for use in forensic science. Forensic SciInt Genet 13:147–153. https://doi.org/10.1016/j.fsigen.2014.07.011
Lewontin RC (1988) On measures of gametic disequilibrium. Genetics 120(3):849–852
Shi YY, He L (2005) SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 15(2):97–98. https://doi.org/10.1038/sj.cr.7290272
Liu Z, Gao Z, Wang J, Shi J, Liu J, Chen D, Li W, Guo J, Cheng X, Hao T, Li Z, Li Y, Yan J, Zhang G (2020) A method of identifying the blood contributor in mixture stains through detecting blood-specific mRNA polymorphism. Electrophoresis 41(15):1364–1373. https://doi.org/10.1002/elps.202000053
Ziller MJ, Gu H, Müller F, Donaghey J, Tsai LT, Kohlbacher O, De Jager PL, Rosen ED, Bennett DA, Bernstein BE, Gnirke A, Meissner A (2013) Charting a dynamic DNA methylation landscape of the human genome. Nature 500(7463):477–481. https://doi.org/10.1038/nature12433
Watanabe K, Akutsu T, Takamura A, Sakurada K (2016) Evaluation of a blood-specific DNA methylated region and trial for allele-specific blood identification from mixed body fluid DNA. Legal Med (Tokyo, Japan) 22:49–53. https://doi.org/10.1016/j.legalmed.2016.08.004
Watanabe K, Taniguchi K, Akutsu T (2018) Development of a DNA methylation-based semen-specific SNP typing method: a new approach for genotyping from a mixture of body fluids. Forensic SciInt Genet 37:227–234. https://doi.org/10.1016/j.fsigen.2018.09.004
Xie B, Song F, Wang S, Zhang K, Li Y, Luo H (2020) Exploring a multiplex DNA methylation-based SNP typing method for body fluids identification: as a preliminary report. Forensic SciInt 313:110329. https://doi.org/10.1016/j.forsciint.2020.110329
Vidaki A, Daniel B, Court DS (2013) Forensic DNA methylation profiling–potential opportunities and challenges. Forensic SciInt Genet 7(5):499–507. https://doi.org/10.1016/j.fsigen.2013.05.004
McClay JL, Aberg KA, Clark SL, Nerella S, Kumar G, Xie LY, Hudson AD, Harada A, Hultman CM, Magnusson PK, Sullivan PF, Van Den Oord EJ (2014) A methylome-wide study of aging using massively parallel sequencing of the methyl-CpG-enriched genomic fraction from blood in over 700 subjects. Hum Mol Genet 23(5):1175–1185. https://doi.org/10.1093/hmg/ddt511
Florath I, Butterbach K, Müller H, Bewerunge-Hudler M, Brenner H (2014) Cross-sectional and longitudinal changes in DNA methylation with age: an epigenome-wide analysis revealing over 60 novel age-associated CpG sites. Hum Mol Genet 23(5):1186–1201. https://doi.org/10.1093/hmg/ddt531
Lee JW, Choung CM, Jung JY, Lee HY, Lim SK (2018) A validation study of DNA methylation-based age prediction using semen in forensic casework samples. Legal Med (Tokyo, Japan) 31:74–77. https://doi.org/10.1016/j.legalmed.2018.01.005
Funding
This work was supported by the National Natural Science Foundation of China [No.81701868 and No.82030058], the Startup Foundation for Doctors of Shanxi Medical University [BS03201621], and the Key Research and Development (R&D) Projects of Shanxi Province [No.201803D31069].
Author information
Authors and Affiliations
Contributions
The authors Gengqian Zhang and Zeqin Li contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Zeqin Li, Jintao Li, Yidan Li, Na Liu, Feng Liu, and Jianbo Ren. The first draft of the manuscript was written by Zeqin Li and Jintao Li, and all authors commented on previous versions of the manuscript. The authors Jiangwei Yan, Gengqian Zhang, and Keming Yun revised it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee of Shanxi Medical University (No. 2019sll014). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the data in Supplementary Table 6.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Li, J., Li, Y. et al. Development of a multiplex methylation-sensitive restriction enzyme-based SNP typing system for deconvolution of semen-containing mixtures. Int J Legal Med 135, 1281–1294 (2021). https://doi.org/10.1007/s00414-021-02552-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-021-02552-9



