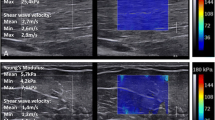
Abstract
Cadaveric rigidity—also referred to as rigor mortis—is a valuable source of information for estimating the time of death, which is a fundamental and challenging task in forensic sciences. Despite its relevance, assessing the level of cadaveric rigidity still relies on qualitative and often subjective observations, and the development of a more quantitative approach is highly demanded. In this context, ultrasound shear wave elastography (US SWE) appears to be a particularly well-suited technique for grading cadaveric rigidity, as it allows non-invasive quantification of muscle stiffness in terms of Young’s modulus (E), which is a widely used parameter in tissue biomechanics. In this pilot study, we measured, for the first time in the literature, changes in the mechanical response of muscular tissues from 0 to 60 h post-mortem (hpm) using SWE, with the aim of investigating its applicability to forensic practice. For this purpose, 26 corpses were included in the study, and the muscle mechanical response was measured at random times in the 0–60 hpm range. Despite the preliminary nature of this study, our data indicate a promising role of SWE in the quantitative determination of cadaveric rigidity, which is still currently based on qualitative and semiquantitative methods. A more in-depth study is required to confirm SWE applicability in this field in order to overcome some of the inherent limitations of the present work, such as the rather low number of cases and the non-systematic approach of the measurements.





Similar content being viewed by others
Abbreviations
- PMI:
-
Post-mortem interval
- SWE:
-
Shear wave elastography
- hpm:
-
Hours post-mortem
References
Madea B (2016) Methods for determining time of death. Forensic Sci Med Pathol 12(4):451–485
Henssge C, Madea B (2004) Estimation of the time since death in the early post-mortem period. Forensic Sci Int 144(2–3):167–175
Rosa MF, Scano P, Noto A, Nioi M, Sanna R, Paribello F, De-Giorgio F, Locci E, d'Aloja E (2015) Monitoring the modifications of the vitreous humor metabolite profile after death: an animal model. Biomed Res Int:627201–627207. https://doi.org/10.1155/2015/627201
Locci E, Scano P, Rosa MF, Nioi M, Noto A, Atzori L, Demontis R, De-Giorgio F, d’Aloja E A metabolomic approach to animal vitreous humor topographical composition: a pilot study. PLoS One 9(5):e97773. https://doi.org/10.1371/journal.pone.0097773 eCollection 2014
Locci E, Stocchero M, Noto A, Chighine A, Natali L, Napoli PE, Caria R, De-Giorgio F, Nioi M, d'Aloja E (2019) A (1)H NMR metabolomic approach for the estimation of the time since death using aqueous humour: an animal model. Metabolomics. 15(5):76. https://doi.org/10.1007/s11306-019-1533-2.6
Ferreira PG, Muñoz-Aguirre M, Reverter F, Sá Godinho CP, Sousa A, Amadoz A, Sodaei R, Hidalgo MR, Pervouchine D, Carbonell-Caballero J, Nurtdinov R, Breschi A, Amador R, Oliveira P, Çubuk C, Curado J, Aguet F, Oliveira C, Dopazo J, Sammeth M, Ardlie KG, Guigó R (2018) The effects of death and post-mortem cold ischemia on human tissue transcriptomes. Nat Commun 9(1):490. https://doi.org/10.1038/s41467-017-02772-x
Zhu Y, Wang L, Yin Y, Yang E (2017) Systematic analysis of gene expression patterns associated with postmortem interval in human tissues. Sci Rep 7(1):5435. https://doi.org/10.1038/s41598-017-05882-0
Nioi M, Napoli PE, Demontis R, Locci E, Fossarello M, d'Aloja E (2018) Morphological analysis of corneal findings modifications after death: a preliminary OCT study on an animal model. Exp Eye Res 169:20–27. https://doi.org/10.1016/j.exer.2018.01.013
Pittner S, Ehrenfellner B, Monticelli FC, Zissler A, Sänger AM, Stoiber W, Steinbacher P (2016) Postmortem muscle protein degradation in humans as a tool for PMI delimitation. Int J Legal Med 130(6):1547–1555
Vain A, Kauppila R, Humal LH, Vuori E (1992) Grading rigor mortis with myotonometry--a new possibility to estimate time of death. Forensic Sci Int 56(2):147–150
Vain A, Kauppila R, Vuori E (1996) Estimation of the breaking of rigor mortis by myotonometry. Forensic Sci Int 79(2):155–161
Martins PA, Ferreira F, Natal Jorge R, Parente M, Santos A (2015) Necromechanics: death-induced changes in the mechanical properties of human tissues. Proc Inst Mech Eng H 229(5):343–349. https://doi.org/10.1177/0954411915581409
De-Giorgio F, Nardini M, Foti F, Minelli E, Papi M, d’Aloja E, Pascali VL, De Spirito M, Ciasca G (2019) A novel method for post-mortem interval estimation based on tissue nano-mechanics. Int J Legal Med 133(4):1133–1139. https://doi.org/10.1007/s00414-019-02034-z
Tian M, Li Y, Liu W, Jin L, Jiang X, Wang X, Ding Z, Peng Y, Zhou J, Fan J, Cao Y, Wang W, Shi Y (2015) The nanomechanical signature of liver cancer tissues and its molecular origin. Nanoscale 7(30):12998–13010. https://doi.org/10.1039/c5nr02192h
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Biomater 3(4):413–438
Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U, Bentires-Alj M, Lim RY, Schoenenberger CA (2012) The nanomechanical signature of breast cancer. Nat Nanotechnol 7(11):757–765. https://doi.org/10.1038/nnano.2012.167
Minelli E, Sassun TE, Papi M, Palmieri V, Palermo F, Perini G, Antonelli M, Gianno F, Maulucci G, Ciasca G, De Spirito M (2018) Nanoscale mechanics of brain abscess: an atomic force microscopy study. Micron. 113:34–40. https://doi.org/10.1016/j.micron.2018.06.012
Minelli E, Ciasca G, Sassun TE, Antonelli M, Palmieri V, Papi M, Maulucci G, Santoro A, Giangaspero F, Delfini R, Campi G, De Spirito M (2017) A fully automated neural network analysis of AFM force-distance curves for cancer tissue diagnosis. Appl Ohys Lett. https://doi.org/10.10603/1.4996300
Kuznetsova TG, Starodubtseva MN, Yegorenkov NI, Chizhik SA, Zhdanov RI (2007) Atomic force microscopy probing of cell elasticity. Micron. 38(8):824–833
Ciasca G, Papi M, Di Claudio S, Chiarpotto M, Palmieri V, Maulucci G, Nocca G, Rossi C, De Spirito M (2015) Mapping viscoelastic properties of healthy and pathological red blood cells at the nanoscale level. Nanoscale. 7(40):17030–17037. https://doi.org/10.1039/c5nr03145a
Ciasca G, Papi M, Minelli E, Palmieri V, De Spirito M (2016) Changes in cellular mechanical properties during onset or progression of colorectal cancer. World J Gastroenterol 22(32):7203–7214. https://doi.org/10.3748/wjg.v22.i32.7203
Perini G, Ciasca G, Minelli E, Papi M, Palmieri V, Maulucci G, Nardini M, Latina V, Corsetti V, Florenzano F, Calissano P, De Spirito M, Amadoro G (2019) Dynamic structural determinants underlie the neurotoxicity of the N-terminal tau 26-44 peptide in Alzheimer’s disease and other human tauopathies. Int J Biol Macromol 141:278–289. https://doi.org/10.1016/j.ijbiomac.2019.08.220
Ciasca G, Pagliei V, Minelli E, Palermo F, Nardini M, Pastore V, Papi M, Caporossi A, De Spirito M, Minnella AM (2019) Nanomechanical mapping helps explain differences in outcomes of eye microsurgery: a comparative study of macular pathologies. PLoS One 14(8):e0220571. https://doi.org/10.1371/journal.pone.0220571
Ciasca G, Sassun TE, Minelli E, Antonelli M, Papi M, Santoro A, Giangaspero F, Delfini R, De Spirito M (2016) Nano-mechanical signature of brain tumours. Nanoscale. 8(47):19629–19643
Ciasca G, Mazzini A, Sassun TE, Nardini M, Minelli E, Papi M, Palmieri V, de Spirito M (2019) Efficient spatial sampling for AFM-based cancer diagnostics: a comparison between neural networks and conventional data analysis. Condens Matter 4. https://doi.org/10.3390/condmat4020058
(2013) Introductory biomechanics: from cells to organisms. Choice Rev Online. https://doi.org/10.5860/choice.45-1476
Choi YJ, Lee JH, Baek JH (2015) Ultrasound elastography for evaluation of cervical lymph nodes. Ultrasonography 34(3):157–164. https://doi.org/10.14366/usg.15007
Domenichini R, Pialat JB, Podda A, Aubry S (2017) Ultrasound elastography in tendon pathology: state of the art. Skelet Radiol 46(12):1643–1655. https://doi.org/10.1007/s00256-017-2726-2 Review
Felicani C, De Molo C, Stefanescu H, Conti F, Mazzotta E, Gabusi V, Nardi E, Morselli-Labate AM, Andreone P, Serra C (2018) Point quantification elastography in the evaluation of liver elasticity in healthy volunteers: a reliability study based on operator expertise. J Ultrasound 21(2):89–98. https://doi.org/10.1007/s40477-018-0300-y
Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C (2016) Ultrasound shear wave elastography for liver disease. A critical appraisal of the many actors on the stage. Ultraschall Med 37(1):1–5. https://doi.org/10.1055/s-0035-1567037
Correas JM, Drakonakis E, Isidori AM, Hélénon O, Pozza C, Cantisani V, Di Leo N, Maghella F, Rubini A, Drudi FM, D'ambrosio F (2014) Reprint of “Update on ultrasound elastography: miscellanea. Prostate, testicle, musculo-skeletal”. Eur J Radiol 83(3):442–449. https://doi.org/10.1016/j.ejrad.2014.01.018
Caliskan E, Ozturk M, Bayramoglu Z, Comert RG, Adaletli I (2018) Evaluation of parotid glands in healthy children and adolescents using shear wave elastography and superb microvascular imaging. Radiol Med 123(9):710–718. https://doi.org/10.1007/s11547-018-0897-0
Vola EA, Albano M, Di Luise C, Servodidio V, Sansone M, Russo S, Corrado B, Servodio Iammarrone C, Caprio MG, Vallone G (2018) Use of ultrasound shear wave to measure muscle stiffness in children with cerebral palsy. J Ultrasound 21(3):241–247. https://doi.org/10.1007/s40477-018-0313-6
Lee SS, Gaebler-Spira D, Zhang LQ, Rymer WZ, Steele KM (2016) Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin Biomech (Bristol, Avon) 31:20–28. https://doi.org/10.1016/j.clinbiomech.2015.10.006
Pichiecchio A, Alessandrino F, Bortolotto C, Cerica A, Rosti C, Raciti MV, Rossi M, Berardinelli A, Baranello G, Bastianello S, Calliada F (2018) Muscle ultrasound elastography and MRI in preschool children with Duchenne muscular dystrophy. Neuromuscul Disord 28(6):476–483. https://doi.org/10.1016/j.nmd.2018.02.007
Bortolotto C, Lungarotti L, Fiorina I, Zacchino M, Draghi F, Calliada F (2017) Influence of subjects’ characteristics and technical variables on muscle stiffness measured by shear wave elastosonography. J Ultrasound 20(2):139–146. https://doi.org/10.1007/s40477-017-0242-9 eCollection 2017 Jun
Bortolotto C, Turpini E, Felisaz P, Fresilli D, Fiorina I, Raciti MV, Belloni E, Bottinelli O, Cantisani V, Calliada F (2017) Median nerve evaluation by shear wave elastosonography: impact of “bone-proximity” hardening artifacts and inter-observer agreement. J Ultrasound 20(4):293–299. https://doi.org/10.1007/s40477-017-0267-0 eCollection 2017 Dec
Lucidarme D, Foucher J, Le Bail B, Vergniol J, Castera L, Duburque C, Forzy G, Filoche B, Couzigou P, de Lédinghen V (2009) Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 49(4):1083–1089. https://doi.org/10.1002/hep.22748
Choi SY, Jeong WK, Kim Y, Kim J, Kim TY, Sohn JH (2014) Shear-wave elastography: a noninvasive tool for monitoring changing hepatic venous pressure gradients in patients with cirrhosis. Radiology. 273(3):917–926. https://doi.org/10.1148/radiol.14140008
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Team RDC, R Development Core Team R (2016) R: a language and environment for statistical computing. R Found Stat Comput. https://doi.org/10.1007/978-3-540-74686-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Research Ethics Committee (N. 0027029/20).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
QQplot of the ten consecutive measurements carried out by operator 1 at each PMI on each corpse. For the sake of clarity, the corpse and the time are indicated in the grey band. As an example, case26-5PMI indicates the measure carried out by the operator on case 26, 5 hours post mortem (PDF 93 kb)
Figure S2
QQplot of the ten consecutive measurements carried out by operator 2 at each PMI on each corpse. (PDF 88 kb)
Figure S3
box plot analysis of the ten measurements that were acquired by the US spectrometer for each data point. Data are arranged in a matrix of plots. In each plot, we show the single measurements that were performed by the US spectrometer when used by operator 1 (yellow) and operator 2 (cyan). The result of t-tests for unpaired samples between the two operators is shown within the text. (PDF 185 kb)
Figure S4
Young’s modulus (E) as a function of age at the different rigor-mortis stages. A linear curve is fitted to the data, and the corresponding regression equation is reported in the plot inset. (PDF 31 kb)
Figure S5
Time evolution of Young’s modulus (E) of the gluteus muscle from 0 to 60 hpm for the analysed cadavers measured by the two operators, together with the corresponding mean difference curve. (PDF 117 kb)
Figure S6
comparison between BMI-adjusted and BMI- & Age-adjusted E values as a function of the PMI (PDF 64 kb)
Table S2
Summary of all ten measures performed by each operator at each time point. (PDF 300 kb)
Rights and permissions
About this article
Cite this article
De-Giorgio, F., Ciasca, G., D’Amico, R. et al. An evaluation of the objectivity and reproducibility of shear wave elastography in estimating the post-mortem interval: a tissue biomechanical perspective. Int J Legal Med 134, 1939–1948 (2020). https://doi.org/10.1007/s00414-020-02370-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-020-02370-5