Abstract
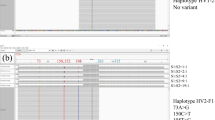
We evaluated whether the number of contributors to mixed DNA samples can be estimated by analyzing the D-loop of mitochondrial DNA using massively parallel sequencing. The A- (positions 16,209–16,400) and B- (positions 30–284) amplicons in hypervariable regions 1 and 2, respectively, were sequenced using MiSeq with 2 × 251 cycles. Sequence extraction and trimming were performed using CLC Genomics Workbench 11 and the number of observed haplotypes was counted for each amplicon type using Microsoft Excel. The haplotype ratios were calculated by dividing the number of counted reads of the corresponding haplotype by the total number of sequence reads. Haplotypes that were over the threshold (5%) were defined as positive haplotypes. The number of larger positive haplotypes in either of the two amplicon types was defined as the number of contributors. Samples were collected from seven individuals. Seventeen mixed samples were prepared by mixing DNA from two to five contributors at various ratios. The number of contributors was correctly estimated from almost all of the mixed samples containing equal amounts of DNA from two to five people. In mixed samples of two or three people, the minor components were detected down to a ratio of 20:1 or 8:2:1. However, heteroplasmy, base deletions, and sharing of the same haplotypes caused incorrect estimations of the number of contributors. Although this method still has room for improvement, it may be useful for estimating the number of contributors in a mixed sample, as it does not rely on forensic mathematics.
Similar content being viewed by others
References
Bleka Ø, Storvik G, Gill P (2016) EuroForMix: an open source software based on a continuous model to evaluate STR DNA profiles from a mixture of contributors with artefacts. Forensic Sci Int Genet 21:35–44. https://doi.org/10.1016/j.fsigen.2015.11.008
Manabe S, Morimoto C, Hamano Y, Fujimoto S, Tamaki K (2017) Development and validation of open-source software for DNA mixture interpretation based on a quantitative continuous model. PLoS ONE 12(11):e0188183. https://doi.org/10.1371/journal.pone.0188183
Marsden CD, Rudin N, Inman K, Lohmueller KE (2016) An assessment of the information content of likelihood ratios derived from complex mixtures. Forensic Sci Int Genet 22:64–72. https://doi.org/10.1016/j.fsigen.2016.01.008
Mitchell AA, Tamariz J, O’Connell K, Ducasse N, Budimlija Z, Prinz M, Caragine T (2012) Validation of a DNA mixture statistics tool incorporating allelic drop-out anddrop-in. Forensic Sci Int Genet 6:749–761. https://doi.org/10.1016/j.fsigen.2012.08.007
Inman K, Rudin N, Cheng K, Robinson C, Kirschner A, Inman-Semerau L, Lohmueller KE (2015) Lab Retriever: a software tool for calculating likelihood ratios incorporating a probability of drop-out for forensic DNA profiles. BMC Bioinformatics 16:298. https://doi.org/10.1186/s12859-015-0740-8
Manabe S, Mori Y, Kawai C, Ozeki M, Tamaki K (2013) Mixture interpretation: experimental and simulated reevaluation of qualitative analysis. Legal Med 15:66–71. https://doi.org/10.1016/j.legalmed.2012.09.001
Parson W, Ballard D, Budowle B, Butler JM, Gettings KB, Gill P, Gusmão L, Hares DR, Irwin JA, King JL, Knijff P, Morling N, Prinz M, Schneider PM, Neste CV, Willuweit S, Phillips C (2016) Massively parallel sequencing of forensic STRs: considerations of the DNA commission of the International Society for Forensic Genetics (ISFG) on minimal nomenclature requirements. Forensic Sci. Int. Genet. 22:54–63. https://doi.org/10.1016/j.fsigen.2016.01.009
Warshauer DH, Lin D, Hari K, Jain R, Davis C, LaRue B, King JL, Budowle B (2013) STRait Razor: a length-based forensic STR allele-calling tool for use with second generation sequencing data. Forensic Sci Int Genet 7:409–417. https://doi.org/10.1016/j.fsigen.2013.04.005
Jäger AC, Alvarez ML, Davis CP, Guzmán E, Han Y, Way L, Walichiewicz P, Silva D, Pham N, Caves G, Bruand J, Schlesinger F, Pond SJK, Varlaro J, Stephens KM, Holt CL (2017) Developmental validation of the MiSeq FGx forensic genomics system for targeted next generation sequencing in forensic DNA casework and database laboratories. Forensic Sci Int Genet 28:52–70. https://doi.org/10.1016/j.fsigen.2017.01.011
Guo F, Zhou Y, Song H, Zhao J, Shen H, Zhao B, Liu F, Jiang X (2016) Next generation sequencing of SNPs using the HID-Ion AmpliSeqTM Identity Panel on the Ion Torrent PGMTM platform. Forensic Sci Int Genet 25:73–84. https://doi.org/10.1016/j.fsigen.2016.07.021
Pakstis AJ, Speed WC, Fang R, Hyland FCL, Furtado MR, Kidd JR, Kidd KK (2010) SNPs for a universal individual identification panel. Hum Genet 127:315–324. https://doi.org/10.1007/s00439-009-0771-1
Nakanishi H, Pereira V, Børsting C, Yamamoto T, Tvedebrink T, Hara M, Takada A, Saito K, Morling N (2018) Analysis of mainland Japanese and Okinawan Japanese populations using the precision ID Ancestry Panel. Forensic Sci Int Genet 33:106–109. https://doi.org/10.1016/j.fsigen.2017.12.004
Parson W, Strobl C, Huber G, Zimmermann B, Gomes SM, Souto L, Fendt L, Delport R, Langit R, Wootton S, Lagacé R, Irwin J (2013) Reprint of: Evaluation of next generation mtGenome sequencing using the Ion Torrent Personal Genome Machine (PGM). Forensic Sci Int Genet 7:632–639. https://doi.org/10.1016/j.fsigen.2013.09.007
King JL, LaRue BL, Novroski NM, Stoljarova M, Seo SB, Zeng X, Warshauer DH, Davis CP, Parson W, Sajantila A, Budowle B (2014) High-quality and high-throughput massively parallel sequencing of the human mitochondrial genome using the Illumina MiSeq. Forensic Sci Int Genet 12:128–135. https://doi.org/10.1016/j.fsigen.2014.06.001
Davis C, Peters D, Warshauer D, King J, Budowle B (2015) Sequencing the hypervariable regions of human mitochondrial DNA using massively parallel sequencing: Enhanced data acquisition for DNA samples encountered in forensic testing. Legal Med 17:123–127. https://doi.org/10.1016/j.legalmed.2014.10.004
McElhoe JA, Holland MM, Makova KD, Su MS, Paul IM, Baker CH, Faith SA, Young B (2014) Development and assessment of an optimized next-generation DNA sequencing approach for the mtgenome using the Illumina MiSeq. Forensic Sci Int Genet 13:20–29. https://doi.org/10.1016/j.fsigen.2014.05.007
Mikkelsen M, Frank-Hansen R, Hansen AJ, Morling N (2014) Massively parallel pyrosequencing of the mitochondrial genome with the 454 methodology in forensic genetics. Forensic Sci Int Genet 12:30–37. https://doi.org/10.1016/j.fsigen.2014.03.014
Churchill JD, Stoljarova M, King JL, Budowle B (2018) Massively parallel sequencing-enabled mixture analysis of mitochondrial DNA samples. Int J Legal Med 132:1263–1272. https://doi.org/10.1007/s00414-018-1799-3
Kim H, Erlich HA, Calloway CD (2015) Analysis of mixtures using next generation sequencing of mitochondrial DNA hypervariable regions. Croat Med J 56:208–217. https://doi.org/10.3325/cmj.2015.56.208
Peck MA, Sturk-Andreaggi K, Thomas JT, Oliver RS, Barritt-Ross S, Marshall C (2018) Developmental validation of a Nextera XT mitogenome Illumina MiSeq sequencing method for high-quality samples. Forensic Sci Int Genet 34:25–36. https://doi.org/10.1016/j.fsigen.2018.01.004
Peck MA, Brandhagen MD, Marshall C, Diegoli TM, Irwin JA, Sturk-Andreaggi K (2016) Concordance and reproducibility of a next generation mtGenome sequencing method for high-quality samples using the Illumina MiSeq. Forensic Sci Int Genet 24:103–111. https://doi.org/10.1016/j.fsigen.2016.06.003
Holland MM, Wilson LA, Copeland S, Dimick G, Holland CA, Bever R, McElhoe JA (2017) MPS analysis of the mtDNA hypervariable regions on the MiSeq with improved enrichment. Int J Legal Med 131:919–931. https://doi.org/10.1007/s00414-017-1530-9
Nakahara H, Sekiguchi K, Imaizumi K, Mizuno N, Kasai K (2008) Heteroplasmies detected in an amplified mitochondrial DNA control region from a small amount of template. J Forensic Sci 53:306–311. https://doi.org/10.1111/j.1556-4029.2007.00655.x
Sekiguchi K, Imaizumi K, Matsuda H, Mizuno N, Yoshida K, Senju H, Sato H, Kasai K (2003) MtDNA sequence analysis using capillary electrophoresis and its application to the analysis of mtDNA in hair. Jpn J Sci Tech Iden 7:123–130. https://doi.org/10.3408/jasti.7.123
Thorvaldsdóttir H, Robinson JT, Mesirov JP (2013) Integrative GenomicsViewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14:178–192. https://doi.org/10.1093/bib/bbs017
Santos C, Sierra B, Alvarez L, Ramos A, Fernández E, Nogués R, Aluja MP (2008) Frequency and pattern of heteroplasmy in the control region of human mitochondrial DNA. J Mol Evol 67:191–200. https://doi.org/10.1007/s00239-008-9138-9
Wang DY, Chang CW, Lagacé RE, Calandro LM, Hennessy LK (2012) Developmental validation of the AmpFℓSTR® Identifiler® Plus PCR Amplification Kit: an established multiplex assay with improved performance. J Forensic Sci 57:453–465. https://doi.org/10.1111/j.1556-4029.2011.01963.x
Acknowledgments
The authors thank Dr. Hajime Miyaguchi (National Research Institute of Police Science) for his help with the MiSeq analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained from all of the participants.
Ethics approval
This study was approved by the Ethics Committee of Juntendo University School of Medicine (No. 2016134).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakanishi, H., Fujii, K., Nakahara, H. et al. Estimation of the number of contributors to mixed samples of DNA by mitochondrial DNA analyses using massively parallel sequencing. Int J Legal Med 134, 101–109 (2020). https://doi.org/10.1007/s00414-019-02182-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-019-02182-2