Abstract
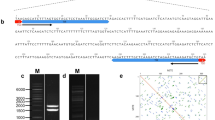
Part of the male population of the wasp Trichogramma kaykai carries a B chromosome that manipulates its host sex ratio in favour of males. The only known repeat on this paternal sex ratio (PSR) chromosome is the 45S rDNA, which includes here five different internal transcribed spacer 2 (ITS2) sequences. In this report, we describe that only part of these ITS2 sequences is transcribed. The absence of transcription of some ITS2 sequences might explain the presence of multiple ITS2 sequences on the PSR chromosome since homogenization of rDNA spacers is thought to occur only in transcribed regions. Analysis of the only other known tandem repeat in Trichogramma, the EcoRI repeat, showed that it is absent from the PSR chromosome, and that the T. kaykai EcoRI repeat has 98 and 77% DNA sequence homology with the T. deion and T. brassicae EcoRI repeats, respectively. The size of the PSR chromosome measures 9 Mbp and is equal to 3.9% of the haploid T. kaykai genome. Finally, fluorescent in situ hybridization with a pool of high and moderate repetitive T. kaykai DNA (C0t-50) revealed only a very few major tandem repeats on the Trichogramma genome and only 45S rDNA on the PSR chromosome.




Similar content being viewed by others
References
Awgulewitsch A, Bünemann H (1986) Isolation of Y-chromosomal repetitive DNA sequences of Drosophila hydei via enrichment of chromosome-specific sequences by heterogeneous hybridization between female and male DNA. J Biochem Biophys Methods 12:37–50
Bennet MD, Leitch IJ, Price HJ, Johnston JS (2003) Comparisons with Caenorhabditis (∼100 Mb) and Drosophila (∼175 Mb) using flow cytometry show genome size in Arabidopsis to be ∼157 Mb and thus ∼25% larger than the Arabidopsis genome initiative estimate of ∼125 Mb. Ann Bot 91:547–557
Beukeboom LW, Reed KM, Werren JH (1992) Effects of deletions on mitotic stability of the paternal-sex-ratio (PSR) chromosome from Nasonia. Chromosoma 102:20–26
Britten RJ, Kohne DE (1968) Repeated sequences in DNA. Science 161:529–540
Camacho JPM, Sharbel TF, Beukeboom LW (2000) B-chromosome evolution. Philos Trans R Soc Lond B Biol Sci 355:163–178
Chang S-B (2004) Thesis: cytogenetic and molecular studies on tomato chromosomes using diploid tomato and tomato monosomic additions in tetraploid potato. Wageningen University, The Netherlands
Cuadrado A, Jouve N (1994) Highly repetitive sequences in B chromosomes of Secale cereale revealed by fluorescence in situ hybridization. Genome 37:709–712
Dhar MK, Friebe B, Koul AK, Gill BS (2002) Origin of an apparent B chromosome by mutation, chromosome fragmentation and specific DNA sequence amplification. Chromosoma 111:332–340
Dobigny G, Ozouf-Costaz C, Bonillo C, Volobouev VT (2002) ‘AgNORs’ are not always true NORs: new evidence in mammals. Cytogenet Genome Res 98:75–77
Dover G (1982) Molecular drive: a cohesive mode of species evolution. Nature 299:111–117
Eickbush DG, Eickbush TH, Werren JH (1992) Molecular characterization of repetitive DNA sequences from a B chromosome. Chromosoma 101:575–583
Elder JF, Turner BJ (1995) Concerted evolution of repetitive DNA sequences in eukaryotes. Q Rev Biol 70:297–320
Gerlach WL, Bedbrook JR (1979) Cloning and characterisation of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7:1869–1885
Gutknecht J, Sperlich D, Bachmann L (1995) A species specific satellite DNA family of Drosophila subsilvestris appearing predominantly in B chromosomes. Chromosoma 103:539–544
Hogge MAF, King PE (1975) The ultrastructure of spermatogenesis in Nasonia vitripennis (Hymenoptera: Pteromalidae). J Submicrosc Cytol 7:81–96
Huigens ME, De Almeida RP, Boons P, Luck RF, Stouthamer R (2004) Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc R Soc Lond B Biol Sci 271:509–516
Jeong G (2004) Evolutionary interactions between sex ratio distorters and their hosts. Ph.D. thesis, Wageningen University, The Netherlands
Landais I, Chavigny P, Castagnone C, Pizzol J, Abad P, Vanlerberghe-Masutti F (2000) Characterization of a highly conserved satellite DNA from the parasitoid wasp Trichogramma brassicae. Gene 255:65–73
Lim KY, Kovarik A, Matyasek R, Bezdek M, Lichtenstein CP, Leitch AR (2000) Gene conversion of ribosomal DNA in Nicotiana tabacum is associated with undermethylated, decondensed and probably active gene units. Chromosoma 109:161–172
López-León MD, Neves N, Schwarzacher T, Heslop-Harrison JS, Hewitt GM, Camacho JPM (1994) Possible origin of a B chromosome deduced from its DNA composition using double FISH technique. Chromosome Res 2:87–92
Marie D, Brown SC (1993) A cytometric exercise in plant DNA histograms, with 2C values for 70 species. Biol Cell 78:41–51
Nardon C, Weiss M, Vieira C, Biémont C (2003) Variation of the genome size estimate with environmental conditions in Drosophila melanogaster. Cytometry Part A 55A:43–49
Peterson DG, Pearson WR, Stack SM (1998) Characterization of the tomato (Lycopersicon esculentum) genome using in vitro and in situ DNA reassociation. Genome 41:346–356
Peterson DG, Schulze SR, Sciara EB, Lee SA, Bowers JE, Nagel A, Jiang N, Tibbitts DC, Wessler SR, Paterson AH (2002) Integration of C0t analysis, DNA cloning, and high-throughput sequencing facilitates genome characterization and gene discovery. Genome Res 12:795–807
Pinto JD, Oatman ER, Platner GR (1986) Trichogramma pretiosum and a new cryptic species occurring sympatrically in southwestern North America (Hymenoptera: Trichogrammatidae). Ann Entomol Soc Am 79:1019–1028
Pinto JD, Stouthamer R, Platner GR, Oatman ER (1991) Variation in reproductive compatibility in Trichogramma and its taxonomic significance (Hymenoptera: Trichogrammatidae). Ann Entomol Soc Am 84:37–46
Pinto JD, Platner GR, Sassaman CA (1993) Electrophoretic study of two closely related species of North American Trichogramma: T. pretiosum and T. deion (Hymenoptera: Trichogrammatidae). Ann Entomol Soc Am 86:702–709
Pinto JD, Stouthamer R, Platner GR (1997) A new cryptic species of Trichogramma (Hymenoptera: Trichogrammatidae) from the Mojave Desert of California as determined by morphological, reproductive and molecular data. Proc Entomol Soc Wash 99:238–247
Puertas MJ (2002) Nature and evolution of B chromosomes in plants: a non-coding but information-rich part of plant genomes. Cytogenet Genome Res 96:1–4
Rasch EM, Cassidy JD, King RC (1975) Estimates of genome size in haplo-diploid species of parasitoid wasps. Abstr Histochem Soc 23:317
Reed KM (1993) Cytogenetic analysis of the paternal sex ratio chromosome of Nasonia vitripennis. Genome 36:157–161
Reed KM, Beukeboom LW, Eickbush DG, Werren JH (1994) Junctions between repetitive DNAs on the PSR chromosome of Nasonia vitripennis: association of palindromes with recombination. J Mol Evol 38:352–362
Reeves A, Tear J (2000) MicroMeasure for Windows, version 3.3. Free program distributed over the Internet from http://www.colostate.edu/Depts/Biology/MicroMeasure
Renault S, Rouleux-Bonnin F, Periquet G, Bigot Y (1999) Satellite DNA transcription in Diadromus pulchellus (Hymenoptera). Insect Biochem Mol Biol 29:103–111
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Stouthamer R, Hu J, Van Kan FJPM, Platner GR, Pinto JD (1999) The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. Biocontrol 43:421–440
Stouthamer R, Van Tilborg M, De Jong JH, Nunney L, Luck RF (2001) Selfish element maintains sex in natural populations of a parasitoid wasp. Proc R Soc Lond B Biol Sci 268:617–622
Van Rijswijk M (2000) Occurrence and molecular identification of Dutch Trichogramma species. M.Sc. thesis, Laboratory of Entomology, Wageningen University, The Netherlands, pp 29.
Van Vugt JJFA (2005) The mode of action, origin and structure of the paternal sex ratio chromosome in the parasitoid wasp Trichogramma kaykai. Ph.D. thesis, Wageningen University, The Netherlands, pp 120
Van Vugt JJFA, Salverda M, De Jong JH, Stouthamer R (2003) The paternal sex ratio chromosome in the parasitic wasp Trichogramma kaykai condenses the paternal chromosomes into a dense chromatin mass. Genome 46:580–587
Wilkes TM, Francki MG, Langridge P, Karp A, Jones RN, Forster JW (1995) Analysis of rye B-chromosome structure using fluorescence in situ hybridization (FISH). Chromosome Res 3:466–472
Zhong X-B, Fransz PF, Wennekes-Van Eden J, Zabel P, Van Kammen A, De Jong JH (1996) High-resolution mapping on pachytene chromosomes and extended DNA fibres by fluorescence in-situ hybridisation. Plant Mol Biol Report 14:232–242
Acknowledgements
We thank Dr. J. S. Johnston from Texas A&M University, USA, and Mr. Patrick Verbaarschot for their help on estimating the genome size. We also thank Mrs. Jannie Wennekes for her help on the FISH of the EcoRI repeat and Ms. Penka Pavlova for measuring the fluorescence intensity of metaphase chromosomes. We also acknowledge the Dutch Science Foundation (NWO-ALW 810.34.006) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Shaw
Rights and permissions
About this article
Cite this article
van Vugt, J.J.F.A., de Nooijer, S., Stouthamer, R. et al. NOR activity and repeat sequences of the paternal sex ratio chromosome of the parasitoid wasp Trichogramma kaykai . Chromosoma 114, 410–419 (2005). https://doi.org/10.1007/s00412-005-0026-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-005-0026-4