Abstract
To fully understand the radiation effects of the atomic bombing of Hiroshima and Nagasaki among the survivors, radiation from neutron-induced radioisotopes in soil and other materials should be considered in addition to the initial radiation directly received from the bombs. This might be important for evaluating the radiation risks to the people who moved to these cities soon after the detonations and probably inhaled activated radioactive “dust.” Manganese-56 is known to be one of the dominant radioisotopes produced in soil by neutrons. Due to its short physical half-life, 56Mn emits residual radiation during the first hours after explosion. Hence, the biological effects of internal exposure of Wistar rats to 56Mn were investigated in the present study. MnO2 powder was activated by a neutron beam to produce radioactive 56Mn. Rats were divided into four groups: those exposed to 56Mn, to non-radioactive Mn, to 60Co γ rays (2 Gy, whole body), and those not exposed to any additional radiation (control). On days 3, 14, and 60 after exposure, the animals were killed and major organs were dissected and subjected to histopathological analysis. As described in more detail by an accompanying publication, the highest internal radiation dose was observed in the digestive system of the rats, followed by the lungs. It was found that the number of mitotic cells increased in the small intestine on day 3 after 56Mn and 60Co exposure, and this change persisted only in 56Mn-exposed animals. Lung tissue was severely damaged only by exposure to 56Mn, despite a rather low radiation dose (less than 0.1 Gy). These data suggest that internal exposure to 56Mn has a significant biological impact on the lungs and small intestine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After the atomic bombing of Hiroshima and Nagasaki, Japan, initial radiation directly produced during or shortly after the explosions and residual radiation contributed towards a radiation exposure of the survivors (Imanaka et al. 2008). There are two sources of residual radiation: (1) neutron-activated radioisotopes from materials on the ground and (2) radioactive fallout containing fission products and residual fissile materials from the bombs. Understanding the former is particularly important for evaluating the risks to those people who moved to these cities soon after the detonations and might have inhaled radioactive dust (Kerr et al. 2013, 2015; Imanaka et al. 2008). Such individuals were reported to suffer from various syndromes similar to acute radiation effects (Imanaka et al. 2012). The neutron-induced radioisotopes include 24Na, 28Al, 31Si, 32P, 32Cl, 42K, 45Ca, 48Sc, and 56Mn, among others (Tanaka et al. 2008). In terms of radiation exposure, 56Mn (physical half-life: 2.58 h), which emits both β particles and γ rays, is one of the most important radioisotopes produced after the atomic bomb explosion in Hiroshima.
To understand the radiation effect of 56Mn, neutron-activated 56MnO2 powder was sprayed over rats, and its biological effects were evaluated. The highest doses of internal irradiation were recorded in the digestive system, followed by the lungs (Stepanenko et al. 2016a, b). Here the results of histological examinations in rats exposed to 56Mn are reported.
Materials and methods
Animals
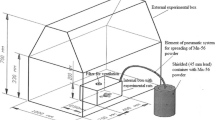
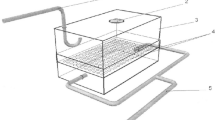
Five-month-old male Wistar rats were obtained from Karaganda State Medical University, Kazakhstan. They were maintained with free access to basal diet and tap water. In Experiment 1, rats were divided into four groups, with six rats for the 56Mn group and three rats per group for the Mn, 60Co, and control groups. The 56Mn and Mn groups were exposed to 56MnO2 and non-radioactive MnO2, respectively. The 60Co group received 2 Gy of external 60Co γ-ray irradiation. Three rats of the 56Mn group were killed for dosimetry 3.5–4 h after the exposure. One rat from each group was killed on days 3, 14, and 60. In Experiment 2, the exposure was repeated with 12 rats in the 56Mn group and nine rats each in the Mn, 60Co, and control groups. Three rats of the 56Mn group were killed for dosimetry 3.5–4 h after the exposure. Then, three rats from each group were killed and examined on days 3, 14, and 60 after the irradiation (Table 1).
Irradiation and dosimetry of each organ of rats
Details of irradiation using 56Mn and the corresponding internal dose estimation have been described in Stepanenko et al. 2016a and Stepanenko et al. 2016b. In brief, 56MnO2 was obtained by neutron activation of 100 mg of MnO2 powder using the Baikal-1 nuclear reactor at Kurchatov, Kazakhstan. A thermal neutron fluence of 4 × 1014 n/cm2 was applied to produce 2.74 × 108 Bq of radioactivity of 56Mn. The activated MnO2 powder was sprayed into sealed boxes containing six rats per box (one box was used for Experiment 1, and two boxes were used for Experiment 2). In Experiment 1, the exposure box was equipped with air filters only, while an additional forced ventilation system was installed to improve animal welfare in the boxes in Experiment 2. The same total activity of 56Mn equal to 2.74 × 108 Bq was used for irradiation in both experiments. The specific activity of 56Mn powder was the same (2.74 × 109 Bq/g) as well. After 1 h, rats were removed from the exposure box(es) into fresh cages, cooled down for 2.5–3 h. Then, three of the animals were killed by intraperitoneal injection of an excessive dose of pentobarbital. A piece of each organ was dissected, weighted and put into a vial. The radioactivity of 56Mn in samples of each organ was measured with a gamma spectrometer. The absorbed fractions from beta and gamma irradiation of each organ as well as the whole body were calculated based on the Monte Carlo code (version MCNP-4C) and the mathematical phantom of a rat. Assessment of internal radiation doses was performed by measuring of 56Mn activity in each organ and calculating the absorbed fractions of internal exposure to photons and electrons. Details of the dosimetry are described in an accompanying paper (Stepanenko et al. 2016a).
Whole body γ-ray irradiation of 2 Gy was performed at a dose rate of 2.6 Gy/min using a Teragam K-2 unit (UJP Praha, Praha-Zbraslav, Czech Republic).
Pathology
The liver, heart, kidney, trachea, lungs, tongue, esophagus, stomach, small intestine, eyes, and skin were dissected, fixed in 10 % formalin, and embedded in paraffin. Sections of 4 μm thickness were prepared and stained with hematoxylin and eosin (HE). For scoring of mitotic cells in the intestinal crypt, good longitudinal sections of the crypt that aligned with other crypts and contained crypt lumen were selected. At least 30 sections per rat were examined under a light microscope, as described previously (Matsuu et al. 2000; Matsuu-Matsuyama et al. 2010). For pathological examination of the lung tissue, the grades of hemorrhage, emphysema, inflammation (inflammatory cell population), expression of lymphoid follicle, and alveolar wall hypertrophy were scored from “−” to “+++” (Shichijo et al. 2000, 2007).
Statistical analysis
All values were expressed as the mean ± standard error (SE). Mann–Whitney’s U test was applied to evaluate the statistical significance of difference between groups.
Results
Radiation doses due to 56Mn exposure
The radiation dose received from 56Mn varied among different organs (Table 2). Although the initial activities of neutron-activated MnO2 powder were similar in Experiments 1 and 2, the radiation doses of each organ received in Experiment 2 were substantially lower than those received in Experiment 1, i.e., the small intestine received 1330 mGy in Experiment 1 while 150 mGy in Experiment 2. However, the distribution of dose values between tissues was similar in the two experiments, being very high in the digestive system, followed by the lungs and skin. Details are given in Stepanenko et al. (2016a).
Pathological findings
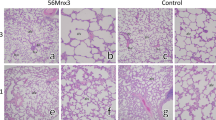
The rats appeared to remain healthy during the study, in both experiments; no deaths were recorded. Compared to the control groups, there were no macropathological changes in the 56Mn, Mn, or 60Co groups. Exposure-related histological changes were noted only in the small intestines and lungs. The number of mitotic cells per crypt in the small intestine is summarized in Table 3 (see also Fig. 1). The number increased in both the 56Mn and 60Co groups on day 3 after the exposure. While this number returned to the control level by day 14 in the 60Co group, it remained high on day 14 in the 56Mn group of Experiment 2 and high on day 60 in the 56Mn group of Experiment 1 as well.
Table 4 summarizes the histological changes in the lungs. In Experiment 1, damage to the lung tissue, including hemorrhage, emphysema, and inflammation, was evident on days 3 and 14 in the 56Mn group (Fig. 2). 60Co exposure, on the other hand, did not cause any significant changes in the lung tissue. The effect of 56Mn on the lungs in Experiment 2 was not as pronounced as that in Experiment 1, but hemorrhage and emphysema were still noted in the 56Mn group.
Discussion
In the present study, two independent experiments were performed with laboratory rats exposed to neutron-activated 56MnO2 powder. Although the absorbed doses of internal irradiation received from 56Mn were rather low in Experiment 2, the observed biological effects were consistent in both the experiments. The lungs were severely damaged by 56Mn, with histological changes, including hemorrhage and emphysema, being present 2 weeks after the exposure. In the small intestine, mitosis was enhanced for an extended period after exposure to 56Mn.
These results might demonstrate that, to understand the radiation effects among the survivors of the atomic bombing of Hiroshima and Nagasaki, it is important to include the effects of residual radiation in addition to those of the initial radiation directly received from the bombs. Residual radiation consists of neutron-induced radioisotopes on the ground and radioactive fallout from the bomb, known as “black rain” (Imanaka et al. 2008). In terms of radiation dose, among the neutron-induced radioisotopes, 56Mn is a dominant radioisotope produced during the atomic bomb explosion (Tanaka et al. 2008). Since 56Mn and the other neutron-activated radioisotopes were present in dust after the bombings, people might have inhaled these radioactive materials and been internally exposed to the radiation. It is important to note that individuals who returned early to Hiroshima and Nagasaki after the atomic bombing were reported to suffer from the symptoms of acute radiation effects (Imanaka et al. 2012). The present international study aimed to investigate the effects of exposure to major residual radioactive particles found in the dust after the atomic bomb explosion by carrying out an animal experiment on the exposure of 56Mn powder to Wistar rats. Although two experiments were performed using the same initial total and specific activity of powdered 56Mn, the absorbed doses due to incorporated 56Mn were substantially higher in Experiment 1 than in Experiment 2. Note, however, that the activity during the irradiation was not measured. Particle size of MnO2 powder was measured and a similar size distribution was found for both experiments ranging from 4 to 10 μm in diameter. The difference between the two experiments was in the ventilation system. In Experiment 2, a forced ventilation system was installed in addition to air filters in the exposure boxes. This may have affected the absorbed doses of internal radiation. Nevertheless, the doses received by the skin were similar in both experiments, which may suggest that the 56MnO2 powder was not taken up very well by either inhalation or ingestion in the rats in Experiment 2, probably due to the difference in ventilation. Note that although the highest radiation doses were observed in the digestive system of the animals in both experiments, this may not be the case in humans because rats ingest a lot of radioactive powder during grooming. The higher radiation doses found in lung tissue, on the other hand, represent an exposure route in humans who inhale dust containing radioactive particles.
It is well known that ionizing radiation increases the occurrence of apoptosis in the crypts of the small intestine, being observed within a period of 3–6 h in rodents (Becciolini et al. 1986; Potten and Grant 1998; Matsuu et al. 2010). The mitotic index, on the other hand, was reported to gradually increase and peaked at 3–4 days after the exposure (Potten and Grant 1998). This coincided with the present data showing increases in mitotic cell numbers on day 3 in both the 56Mn and 60Co groups. Interestingly, in Experiment 2, an increase in mitosis was still observed on day 14 after exposure to 56Mn, while it returned to the control level in the 60Co group, suggesting that the effects of internal radiation of 56Mn were more persistent. However, an increase in mitosis was not found on day 14 but on day 60 in Experiment 1. Further studies are needed to determine whether this effect was due to the difference in radiation dose.
It is also well known that radiation exposure of the lungs can induce “radiation pneumonitis” in laboratory animals, at lung doses above 8 Gy (Coggle et al. 1986; Ward et al. 1992). Studies in rats showed that a thorax irradiation of 20 Gy did not induce any short-term histological changes in the lungs, although the irradiated lungs developed focal exudative lesions after 2 months and then reparative fibrotic lesions by 6 months (Travis et al. 1977; Down 1986). Lung function was also damaged by irradiation with a single dose of 12 Gy (Eerde et al. 2001). These data are consistent with findings of the present pathological examination of the lung tissue in the 60Co group (2 Gy), which did not show any significant changes. In contrast to the external γ-radiation, the internal exposure to 56Mn induced pathological changes, including hemorrhage and emphysema, although the radiation doses were low: 100 mGy in Experiment 1 and 30 mGy in Experiment 2. The stronger biological effects of exposure to 56Mn may be due to its β emissions. Whether these initial pathological changes lead to any long-term lesions should be investigated in the future. Previous studies investigating the effects of inhaled radioactive plutonium, an α-emitter, showed that rats died with severe pulmonary edema at higher radiation doses, while radiation pneumonitis and emphysema developed at lower doses (Lundgren et al. 1981; Dagle and Sanders 1984; Scott et al. 1990). In the present study, occasional inflammations were observed in the lung in control group. This is probably because all of the animals were maintained in the conventional facility since the radiation exposure took place in the non-SPF (Specific-Pathogen Free) condition due to the technical limitation at the nuclear reactor.
Manganese is well known for its neurotoxicity (Crossgrove and Zheng 2004; O’Neal and Zheng 2015). In humans, Mn overexposure induces symptoms similar to those of Parkinson’s disease, although animal studies suggested that the mechanisms underlying toxicity are different (O’Neal and Zheng 2015). Manganese, particularly when administered by inhalation, may be toxic to the lung tissue, although results from epidemiological studies investigating the relationship between exposure to Mn and pulmonary diseases do not provide consistent results (Bergström 1977). Experimental studies have suggested that Mn exposure leads to a primary inflammatory reaction in the respiratory tract (Adkins et al. 1980; Camner et al. 1985). In the present study, there were no signs of toxicity by non-radioactive Mn, although a high dosage of MnO2 (100 mg) was sprayed into the exposure boxes. Since MnO2 powder was sprayed into the boxes without using any apparatus to agitate the powder or produce an aerosol, and because the exposure period was only 1 hour, the total amount of MnO2 inhaled by any rat would have been limited. Therefore, histological changes observed in the 56Mn group were probably the result of radiation exposure and not of chemical toxicity.
Conclusion
This study investigated the effects of radiation exposure by 56MnO2 powder in male Wistar rats over 60 days. Although whole body radiation doses from 56Mn were relatively low, higher internal doses were noted in the small intestine and lungs, in addition to significant pathological changes that were more severe and prolonged than the effects of 60Co γ irradiation. These data may hint towards a potential risk of internal exposure to 56Mn, which might have existed in airborne dust after the atomic bomb explosions over Hiroshima and Nagasaki, Japan.
References
Adkins B, Luginbuhl GH, Gardner DE (1980) Acute exposure of laboratory mice to manganese oxide. Am Ind Hyg Assoc J 41:494–500
Becciolini A, Cremonini D, Fabbrica D, Balzi M (1986) Cell proliferation and differentiation in the small intestine after irradiation with multiple fractions. Acta Radiol Oncol 25:51–56
Bergström R (1977) Acute pulmonary toxicity of manganese dioxide. Scan J Work Environ Health 3(Suppl 1):1–41
Camner P, Curstedt T, Jarstrand C, Johannsson A, Robertson B, Wiernik A (1985) Rabbit lung after inhalation of manganese chloride: a comparison with the effects of chlorides of nickel, cadmium, cobalt, and copper. Environ Res 38:301–309
Coggle JE, Lambert BE, Moores SR (1986) Radiation effects in the lung. Environ Health Persp 70:261–291
Crossgrove J, Zheng W (2004) Manganese toxicity upon overexposure. NMR Biomed 17:544–553
Dagle GE, Sanders CL (1984) Radionuclide injury to the lung. Environ Health Persp 55:129–137
Down JD (1986) The nature and relevance of late lung pathology following localised irradiation of the thorax in mice and rats. Brit J Cancer Suppl 7:330–332
Eerde MRV, Kampinga HH, Szabo BG, Vujaskovic Z (2001) Comparison of three rat strains for development of radiation-induced lung injury after hemithoracic irradiation. Radiother Oncol 58:313–316
Imanaka T, Endo S, Tanaka K, Shizuma K (2008) Gamma-ray exposure from neutron-induced radionuclides in soil in Hiroshima and Nagasaki based on DS02 calculations. Radiat Environ Bioph 47:331–336
Imanaka T, Endo S, Kawano N, Tanaka K (2012) Radiation exposure and disease questionnaires of early entrants after the Hiroshima bombing. Radiat Prot Dosim 149:91–96
Kerr GD, Egbert SD, Al-Nabulsi I, Beck HL, Cullings HM, Endo S, Hoshi M, Imanaka T, Kaul DC, Maruyama S, Reeves GI, Rühm W, Sakaguchi A, Simon SL, Spriggs GD, Stram DO, Tonda T, Weiss JF, Weitz RL, Young RW (2013) Workshop report on atomic bomb dosimetry residual radiation exposure: recent research and suggestions for future studies. Health Phys 105:140–149
Kerr GD, Egbert SD, Al-Nabulsi I, Bailiff IK, Beck HL, Belukha IG, Cockayne JE, Cullings HM, Eckerman KF, Granovskaya E, Grant EJ, Hoshi M, Kaul DC, Kryuchkov V, Mannis D, Ohtaki M, Otani K, Shinkarev S, Simon SL, Spriggs GD, Stepanenko VF, Stricklin D, Weiss JF, Weitz RL, Woda C, Worthington PR, Yamamoto K, Young RW (2015) Workshop report on atomic bomb dosimetry–review of dose related factors for the evaluation of exposures to residual radiation at Hiroshima and Nagasaki. Health Phys 109:582–600
Lundgren DL, Damon EG, Diel JH, Hahn FF (1981) The deposition, distribution and retention of inhaled 234PuO2 in the lungs of rats with pulmonary emphysema. Health Phys 40:231–235
Matsuu M, Shichijo K, Nakamura Y, Ikeda Y, Naito S, Ito M, Okaichi K, Sekine I (2000) The role of the sympathetic nervous system in radiation-induced apoptosis in jejunal crypt cells of spontaneously hypertensive rats. J Radiat Res 41:55–65
Matsuu-Matsuyama M, Nakashima M, Shichijo K, Okaichi K, Nakayama T, Sekine I (2010) Basic fibroblast growth factor suppresses radiation-induced apoptosis and TP53 pathway in rat small intestine. Radiat Res 174:52–61
O’Neal SL, Zheng W (2015) Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep 2:315–328
Potten CS, Grant HK (1998) The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Brit J Cancer 78:993–1003
Scott BR, Hahn FF, Snipes MB, Newton GJ, Eidson AF, Mauderly JL, Boecker BB (1990) Predicted and observed early effects of combined alpha and beta lung irradiation. Health Phys 59:791–805
Shichijo K, Gottfried M, Sekine I, Pappas TN (2000) Dextran sulfate sodium-induced colitis in immunodeficient rats. Dig Dis Sci 45:2320–2326
Shichijo K, Shin T, Wen CY, Nakayama T, Nakashima M, Kaimovich AG, Matsuyama M, Sekine I (2007) Expression of apoptotic epithelial cells in biopsy specimens of patients with colitis. Dig Dis Sci 52:2037–2043
Stepanenko V, Rakhypbekov T, Otani K, Endo S, Satoh K, Kawano N, Shichijo K, Nakashima M, Takatsuji T, Sakaguchi A, Kato H, Onda Y, Fujimoto N, Toyoda S, Sato H, Dyussupov A, Chaizhunusova N, Sayakenov N, Uzbekov D, Saimova A, Shabdarbaeva D, Skakov M, Vurim A, Gnyrya V, Azimkhanov A, Kolbayenkov A, Zhumadilov K, Kairikhanova Y, Kaprin A, Galkin V, Ivanov S, Kolyzhenkov T, Petukhov A, Yaskova E, Belukha I, Khailov A, Skvortsov V, Ivannikov A, Akhmedova U, Bogacheva V, Hoshi M (2016a) Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats—Part 1: Dosimetry. Radiat Environ Biophys. doi:10.1007/s00411-016-0678-x
Stepanenko VF, Rakhypbekov TK, Kaprin AD, Ivanov SA, Otani K, Endo S, Satoh K, Kawano N, Takatsuji T, Nakashima M, Shichijo K, Sakaguchi A, Kato H, Onda Y, Fujimoto N, Toyoda S, Sato H, Kolyzhenkov TV, Petukhov AD, Dyussupov AA, Chaizhunusova NZ, Sayakenov NB, Uzbekov DE, Saimova AZ, Shabdarbaeva DM, Pivina LN, Skakov MK, Vurim AD, Gnyrya VS, Azimkhanov AC, Kolbayenkov AN, Zhumadilov KS, Kairkhanova YO, Yaskova EK, Belukha IG, Skvortsov VG, Ivannikov AI, Khailov AM, Akhmedova UA, Bogacheva VV, Anokhin YN, Orlenko SP, Hoshi M (2016b) Irradiation of experimental animals by neutron activated dust: development and realization of the method—first results of international multicenter study. Radiat Risk 25:111–125
Tanaka K, Endo S, Imanaka T, Shizuma K, Hasai H, Hoshi M (2008) Skin dose from neutron-activated soil for early entrants following the A-bomb detonation in Hiroshima: contribution from β and γ rays. Radiat Environ Bioph 47:323–330
Travis EL, Harley RA, Fenn JO, Klobukowski CJ, Hargrove HB (1977) Pathologic changes in the lung following single and multi-fraction irradiation. Int J Radiat Oncol 2:475–490
Ward WF, Kim YT, Molteni A, Ts’ao C, Hinz JM (1992) Pentoxifylline does not spare acute radiation reactions in rat lung and skin. Radiat Res 129:107–111
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Semey State Medical University, Kazakhstan, and Grant-in-Aids (#26257501 and #23510064) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest
Author Kazuko Shichijo declares that she has no conflict of interest; Author Nariaki Fujimoto declares that he has no conflict of interest; Author Darkhan Uzbekov declares that he has no conflict of interest; Author Ynkar Kairkhanova declares that she has no conflict of interest; Author Aisulu Saimova declares that she has no conflict of interest; Author Nailya Chaizhunusova declares that she has no conflict of interest; Author Nurlan Sayakenov declares that he has no conflict of interest; Author Dariya Shabdarbaeva declares that she has no conflict of interest; Author Nurlan Aukenov declares that he has no conflict of interest; Author Almas Azimkhanov declares that he has no conflict of interest; Author Alexander Kolbayenkov declares that he has no conflict of interest; Author Zhanna Mussazhanova declares that she has no conflict of interest; Author Daisuke Niino declares that he has no conflict of interest; Author Masahiro Nakashima declares that he has no conflict of interest; Author Kassym Zhumadilov declares that he has no conflict of interest; Author Valeriy Stepanenko declares that he has no conflict of interest; Author Masao Tomonaga declares that he has no conflict of interest; Author Tolebay Rakhypbekov declares that he has no conflict of interest; Author Masaharu Hoshi declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The animal experiment was approved by the Animal Experiment Committee of Semey Medical University, Republic of Kazakhstan, and conducted in accordance with the Institutional Guide for Animal Care and Use.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00411-017-0687-4.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shichijo, K., Fujimoto, N., Uzbekov, D. et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats—Part 2: pathological effects. Radiat Environ Biophys 56, 55–61 (2017). https://doi.org/10.1007/s00411-016-0676-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-016-0676-z