Abstract
Glasses quenched from relatively undegassed ocean island magmas erupted from volcanoes at Iceland, Hawaii, the Canary Islands, and Erebus have elevated Fe3+/∑Fe ratios compared to glasses quenched from mid-ocean ridge basalts. This has been ascribed to elevated fO2 of their mantle sources, plausibly due to subducted, oxidized near-surface-derived components in their mantle sources. The basaltic magmas from Reunion Island in the Indian ocean have Sr–Nd-Hf-Pb-Os isotopic compositions suggesting that their mantle sources contain little or no subducted near-surface materials and contain the C/FOZO/PREMA mantle component. To constrain the fO2 of the C/FOZO/PREMA mantle component and test the link between oxidized OIB and recycled surface-derived materials in their sources, we measured major and volatile element abundances and Fe3+/∑Fe ratios of naturally glassy, olivine-hosted melt inclusions from Piton de La Fournaise volcano, La Reunion. We conclude that the fO2 of the mantle source of these Reunion lavas is lower than of the mantle sources of primitive, undegassed magmas from Hawaii, Iceland, the Canary Islands, and Mt. Erebus, and indistinguishable from that of the Indian-ocean upper mantle. This finding is consistent with previous suggestions that the source of Reunion lavas (and the C/FOZO/PREMA mantle component) contains little or no recycled materials and with the suggestion that recycled oxidized materials contribute to the high fO2 of some other OIBs, especially those from incompatible-element-enriched mantle sources. Simple mixing models between oxidized melts of EM1 and HIMU components and relatively reduced melts of DMM can explain the isotopic compositions and Fe3+/∑Fe ratios of lavas from Hawaii, Iceland, the Canary Islands, and Mount Erebus; this model can be tested by study of additional OIB magmas, including those rich in the EM2 component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measurements of the ratio of Fe3+ to total Fe (i.e., Fe3+/[Fe2+ + Fe3+], or Fe3+/∑Fe) in basaltic glasses from ocean island volcanoes (OIBs—Hartley et al. 2017; Shorttle et al. 2015; Brounce et al. 2017; Helz et al. 2017; Moussallam et al. 2014, 2016, 2019) range from low values similar to those of mid-ocean ridge basalts (MORBs – 0.10–0.14; Berry et al. 2018; Zhang et al. 2018) to higher values that exceed those of subduction zone magmas (Fig. 1a; e.g., arc basalts ~ 0.28; Brounce et al. 2014). This range in OIBs is sometimes recorded in samples from the same volcano, from the same island, and/or island group (Brounce et al. 2017; Moussallam et al. 2014; Shorttle et al. 2015; Novella et al. 2020), and even from the same eruption (Hartley et al. 2017; Helz et al. 2017). Moreover, for any given OIB volcano, glasses with the lowest S and H2O concentrations typically have the lowest Fe3+/∑Fe; this correlation of Fe3+/∑Fe ratios with volatile contents has been attributed to reduction that is driven by loss of S during volcanic degassing (Anderson and Wright 1972; Brounce et al. 2017; Carmichael and Ghiorso 1986; Gaillard et al. 2011; Hartley et al. 2017; Helz et al. 2017; Moussallam et al. 2014, 2016, 2019; Shorttle et al. 2015; Novella et al. 2020). Figure 1a shows that the Fe3+/∑Fe ratios of the least degassed glasses range from 0.17 in Icelandic glasses (Hartley et al. 2017; Shorttle et al. 2015; Novella et al. 2020) to as high as 0.30 in glasses from the Canary Islands (Moussallam et al. 2019), corresponding to oxygen fugacities (fO2s) between 0.4 and 2.1 orders of magnitude higher than the quartz-fayalite-magnetite buffer (referred to as ΔQFM values of + 0.4 and + 2.1). The fO2 values of undegassed Hawaiian (Fe3+/∑Fe = 0.18; ΔQFM = + 0.6; Brounce et al. 2017; Moussallam et al. 2016) and Mount Erebus (Fe3+/∑Fe = 0.40; ΔQFM = + 2.0; Moussallam et al. 2014) glasses are between these extremes. In contrast, as summarized in Fig. 1a, wherever they have been sampled, MORB glasses have lower Fe3+/∑Fe ratios, and although the exact average value is debated (ranging from Fe3+/∑Fe = 0.10, Berry et al. 2018, to 0.14, Zhang et al. 2018), there is agreement that MORBs record fO2s of ΔQFM = – 0.7 to + 0.1 (Cottrell and Kelley 2011; O’Neill et al. 2018; Zhang et al. 2018; Birner et al. 2018). The higher fO2s of relatively undegassed glasses from ocean island basalts (OIBs) relative to MORB glasses have been interpreted as having been inherited from their mantle sources and attributed to the presence of recycled surface-derived and oxidized materials in their mantle sources (Brounce et al. 2017; Hartley et al. 2017; Helz et al. 2017; Moussallam et al. 2014, 2016, 2019; Shorttle et al. 2015; Novella et al. 2020).
Summary of the ranges of Fe3+/∑Fe measured via XANES in submarine pillow glass, matrix glass, and glassy inclusions hosted in minerals in a a global collection of MORB, OIB, back-arc basin, and arc basalts, and b Reunion island. Panel a: Circles in panel a are representations of the “most parental” values (least degassed) in each location. The rectangles for MORB correspond to the full range of the data and are from Cottrell and Kelley 2011, recalibrated according to Zhang et al. 2018 and Berry et al. 2018, and the circles represent each study’s preferred average for globally representative sample sets. The values for OIB are from Hartley et al. 2017, Shorttle et al. 2015, (Iceland), Helz et al. 2017, Moussallam et al. 2016, Brounce et al. 2017 (Hawaii), Moussallam et al. 2014 (Erebus), Moussallam et al. 2019 (Canaries), and Reunion (this study, and Gaborieau et al. 2020; shown as G20 on the figure; XANES measurements of two olivine-hosted melt inclusions from a lava flow from Piton des Neiges, the other volcano that makes up Reunion Island). Various arrows and averages reflect the interpretations provided by each study. Gray downward pointing arrows indicate the extent of reduction in Fe3+/∑Fe ratios as the result of degassing, black upward point arrows indicate the extent of oxidation in Fe3+/∑Fe ratios as the result of the entrainment of air into surface exposed lava flows. Pink downward pointing arrow indicates the extent of reduction in Fe3+/∑Fe ratios as the result of diffusive re-equilibration with a degassed host magma (see text). The values for the Mariana trough are from Brounce et al. (2014) and Kelley and Cottrell (2009). The black circle marked “a” corresponds to the average Fe3+/∑Fe of subduction influenced back-arc submarine glasses, and the circle marked “b” corresponds to the average Fe3+/∑Fe of non-subduction influenced back-arc submarine glasses. The values for island arc basalts are from Brounce et al. (2014), Kelley and Cottrell (2012), Kelley and Cottrell (2009).The circle marked “a” is the highest Fe3+/∑Fe measured via XANES in an island arc basaltic glass, and is a single measurement from Augustine volcano (Kelley and Cottrell (2009). The circle marked “b” is the average of the least degassed compositions from the Mariana arc (Brounce et al. 2014). Panel b: This is constructed after Pichavant et al. (2016), with olivine-liquid equilibrium constraints from Burgisser and Bachèlery (2009), bulk rock FeO and Fe2O3 analyses from Burgisser et al. (2015), and olivine-spinel equilibria from Burgisser et al. (2015) and Bureau et al (1998b). The solid black line demonstrates the likely fO2 of Reunion magmas during petrogenesis summarized by Pichavant et al. (2016). The pink box marked “this study” shows the fO2 of least degassed magmas inferred here from XANES analyses of melt inclusions
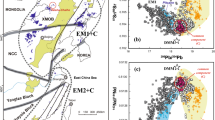
Suites of OIB magmas generally define linear arrays in plots of radiogenic isotope ratios, and these have been interpreted as indicating that the Earth’s mantle contains several isotopically distinct reservoirs or components that make variable contributions to the mantle sources of any given suite. For example, as shown in Fig. 2, Hawaiian and Icelandic magmas have radiogenic isotopic ratios indicating the presence in their source regions of a component characteristic of the “depleted” mantle sources of MORB (DMM; Stracke 2012) plus a “less depleted” component present in many OIB lavas variously referred to as “C”, FOZO, and/or PREMA (Hanan and Graham 1996; Hart et al. 1992; Hauri et al. 1994; Stracke 2012; Stracke et al. 2005; Zindler and Hart 1986). Also inferred to be present in the sources of Hawaiian and Icelandic OIBs (Stracke 2012) are variable amounts of isotopically distinct (and also relatively “enriched”) mantle components with high 87Sr/86Sr but low to moderate 206Pb/208Pb (labeled EM1 and EM2; Fig. 2). Figure 2 also shows that magmas from the Canary Islands and Mount Erebus have isotopic characteristics suggesting mixtures of DMM and C/FOZO/PREMA in their mantle sources, plus variable amounts of another component that is absent in Hawaiian and Icelandic magmas, characterized by distinctive radiogenic Pb isotope ratios (HIMU; Sims et al. 2008; Stracke 2012).
Plots of radiogenic isotopic compositions of oceanic basalts (gray circles: Paciifc and Atlantic MORB; black circles: Indian MORB; yellow, blue, green, red circles: OIB studied via XANES; white circles: other OIB; pink diamonds: Reunion) from the compilation of Stracke et al. (2005), with Erebus isotopic compositions from Sims et al. (2008). The approximate estimates of the isotopic compositions of various proposed mantle components are marked. The proposed ranges of FOZO/PREMA/C are marked according to reference: FOZO H1 (Hart et al. 1992), FOZO H2 (Hauri et al. 1994), FOZO S (Stracke et al. 2005), “C” H&G (Hanan and Graham, 1996). a 87Sr/86Sr versus 206Pb/204Pb and b 208Pb/204Pb versus 206Pb/204Pb
Although definitive links between each isotopic component and specific mantle source lithologies and their provenance are debated, it has been suggested that the EM1, EM2, and HIMU components reflect seafloor sediments, ancient oceanic and/or continental crust and/or lithospheric mantle, and perhaps surface lithologies not currently present at Earth’s surface, recycled into the mantle by subduction and subsequently entrained into mantle plumes (e.g., Boyet et al. 2019; Chauvel et al. 1992; Cordier et al. 2021; Garapic et al. 2015; Hofmann and White 1982; Stracke et al. 2005; Zindler and Hart 1986). The origin of the C/FOZO/PREMA component is less certain than those of the other end members, but it has been variously suggested that (1) it may represent portions of the mantle that many plumes interact with to some degree, located in either the transition zone or the lowermost mantle (Hanan and Graham 1996; Hart et al. 1992; Hauri et al. 1994; Giuliani et al. 2021); (2) it may be present or mixed into the mantle sources of MORB (Stracke et al. 2005); (3) perhaps like the EM1, EM2, and HIMU components, it contains recycled oceanic crust or lithosphere (Castillo et al. 2015; Hanan and Graham 1996; Stracke et al. 2005), and/or (4) it may sample a long-lived mantle component formed by the extraction of melt from a chondritic mantle that contributed to the continental crust (Giuliani et al. 2021). Whatever the ultimate origin of the isotopic fingerprints associated with these mantle end members, the elevated Fe3+/∑Fe ratios relative to MORBs of the least degassed glasses at Iceland, Hawaii, the Canaries, and Mount Erebus have led to the suggestion that the mantle components that contribute the EM1, EM2, and HIMU isotopic characteristics to their magmas may also contribute oxidized signatures, perhaps acquired during exposure of these components to oxygen- and/or H2O-rich conditions at Earth’s surface prior to recycling into the mantle (Brounce et al. 2017; Moussallam 2016, 2019; Shorttle et al. 2015; Stolper and Keller 2018).
The Reunion hotspot, with its two volcanoes, Piton de la Fournaise and Piton des Neiges, is relatively isotopically homogeneous compared to the OIB discussed above: Basalts from both volcanoes have high 208Pb/204Pb and 207Pb/204Pb at a given 206Pb/204Pb; these characteristics define the so-called DUPAL anomaly (Fig. 2b; Dupré and Allègre 1983; Hart 1984) that are shared among OIB and MORB lavas erupted in the Indian Ocean. Taken together, the Pb, Sr (Fig. 2), Nd, and Hf isotopic ratios of Reunion lavas have been used to suggest a dominance of C/FOZO/PREMA in the mantle sources of Reunion lavas (> 90%), with only minor contributions from EM1 (reflected in the DUPAL characteristic of these lavas) and even smaller contributions from DMM (Bosch et al. 2008). The Reunion lavas also have chondritic Th/U (Vlastélic et al. 2006) and unradiogenic 187Os/188Os isotopic ratios (Schiano et al. 2012), suggesting that their mantle sources contain an ancient, slightly trace-element-depleted mantle source that has been minimally influenced by the recycling processes of plate tectonics (Hart 1984; Schiano et al. 2012; Vlastélic et al. 2006; Jackson et al. 2020). Thus, Reunion lavas present the opportunity to study in concentrated form the fO2 of C/FOZO/PREMA without significant complications from recycling of the EM1, EM2, and HIMU mantle components, all of which have been inferrred to contain such recycled and potentially oxidized surface-derived components.
We present here measurements of the major and volatile (H2O, S) element concentrations and Fe3+/∑Fe ratios of naturally glassy melt inclusions from the 2005 eruption of Piton de la Fournaise on Reunion island and use the results to infer fO2 values of undegassed magmas and of the mantle source of this eruption. We use this fO2, in combination with previously reported fO2 constraints for Reunion island (summarized in Fig. 1b, based on Pichavant et al. 2016), to characterize explicitly the fO2 of the widespread C/FOZO/PREMA mantle component and compare it to fO2 levels that have been inferred for other distinguishable mantle components.
Sample description
The samples studied here are from the 2005 eruption of picritic lava flows in the Enclos Fouqué of Piton de la Fournaise volcano, Reunion Island. Large (> 1 mm) olivine grains sampled shortly after eruption were provided for this study by Pascal Richet (IPGP). These samples are similar to those described by Famin et al. (2009). The olivine grains examined in this study contain spherical-to-ovoid naturally glassy melt inclusions ranging in size from ~ 20 μm to ~ 150 μm in diameter, but because of analytical constraints of the Fe-XANES measurements, melt inclusions analyzed here are all > 100 μm in diameter. Most melt inclusions contain vapor bubbles, and some contain spinel crystals and sulfide blebs, though the specific melt inclusions analyzed here do not contain spinels or sulfide blebs. Spinels and sulfides are also present as mineral inclusions (i.e., without significant glass; ~ 10–20 μm in diameter) in the olivine crystals, consistent with their having been present at the time of melt inclusion entrapment and not entirely crystallized from the melt inclusions after entrapment. The olivine grains also contain occasional melt embayments ~ 20 μm wide and 100–200-μm long that do not contain vapor bubbles. Representative photomicrographs and backscattered electron images of the samples are available in supplementary data Tables 1, 2, 3.
Methods
Abundances of major elements, S, and Cl in the melt inclusions and major elements in the olivines were measured using the JEOL JXA-8200 electron microprobe at the California Institute of Technology. Dissolved H2O and CO2 contents in studied glasses were measured via transmission FTIR at the University of California Riverside on a Thermo-Nicolet iS50 spectrometer coupled with a Continuum microscope. We measured Fe3+/∑Fe via Fe-μ-X-ray absorption near edge structure (XANES) spectroscopy at the Advanced Photon Source, Argonne National Laboratory, beamline 13-IDE following collection procedures described by Brounce et al. (2017). Data are reported in Table 1. Analytical details can be found in the supplementary materials and representative pre-edge spectra are shown in Fig. 3.
Results and discussion
Major and minor element compositions of melt inclusions and embayments
The fourteen melt inclusions analyzed in this study are hosted by olivines with compositions ranging from Fo82 to Fo84. To correct the glass analyses for post-entrapment crystallization on the inclusion walls, values from Table 1 were corrected assuming Fe2+/Mg KDol/liq = 0.3 (Roeder and Emslie 1970). We added olivine with a composition in equilibrium with the melt inclusion back to the melt in 0.1 wt% increments until the melt inclusion reached a composition in equilibrium with the host olivine. The amount of olivine added back to the melt inclusions by this correction ranged from 0 to 8.5 wt%, and the corrected melt inclusion compositions contain 6.3–8.5 wt% MgO (Table 1). These MgO contents (and corrected major element compositions more broadly; Fig. 4) are comparable to those obtained by Famin et al. (2009) on glass melt inclusions from the same lava flow using a similar approach and to other melt inclusions from picritic lavas sampled from older eruptions of Piton de la Fournaise (Bureau et al. 1998a, b). We also analyzed three glass embayments partially enclosed by olivines that contain melt inclusions (Reunion-3, Reunion-9, and Reunion-15). These embayment compositions, uncorrected for post-entrapment olivine crystallization, contain 4.9–6.1 wt% MgO.
Plots of major element compositions and H2O contents of olivine-hosted melt inclusions and glassy embayments studied here, compared to reported compositions of olivine-hosted melt inclusions from the same eruption (green circles; Famin et al. 2009), and from older eruptions of the same volcano (yellow circles, Bureau et al. 1998a, b), and SWIR MORB (Birner et al. 2018). a CaO and MgO, b FeO* and MgO, c Al2O3 and MgO, d TiO2 and MgO, e H2O and MgO, and f H2O and K2O
The H2O contents of the studied melt inclusions range from 0.2 to 0.7 wt%, accompanied by a smaller range in K2O (~ 0.6–0.8 wt%; Fig. 4f). This would be consistent with H2O degassing accompanied by minimal crystallization before or during melt inclusion entrapment in host olivine or diffusive loss of H2O from inclusions after entrapment (Le Voyer et al. 2014; Newcombe et al. 2020; Bucholz et al. 2013). As mentioned previously, sulfide blebs are present in some glass inclusions in the same samples (though not in the exact melt inclusions measured here) and as larger inclusions in the host olivines. Sulfur abundances in the glass inclusions are uncorrelated with H2O abundances (Fig. 6a), and—although at best weakly correlated with FeO* (total Fe as FeO)—they are consistent with saturation with molten Fe-rich sulfide (Fig. 5a). Overall, these trends, combined with petrographic observation of sulfide blebs, suggest entrapment as melt inclusions of liquids that were saturated with a sulfide liquid and that S degassing was minimal during or prior to the time of entrapment. However, the S concentrations of the embayments are significantly lower than those of the melt inclusions (71–113 ppm in embayments; 858–1244 ppm in the melt inclusions; Fig. 5a), and H2O concentrations in the embayments were below the FTIR detection limit (which we estimate here to be ~ 0.08 wt% H2O for a doubly polished, ~ 40 μm thick wafer based on the limit below which it begins to be difficult to confidently distinguish signal from noise in IR spectra; Fig. 4e, f). The significant depletion of both H2O and S in the melt embayments relative to the melt inclusions indicates that, in contrast to the melt inclusions, the melt in the embayments degassed nearly to completion upon and/or prior to eruption and emplacement of the lava flow on the surface.
a S and FeO* concentrations of olivine-hosted melt inclusions and glassy embayments. Small gray circles are MORB glasses (Jenner and O’Neill, 2012). Black lines show the calculated S content of silicate melt at sulfide saturation according to the model of O’Neill and Mavrogenes (2002) setting aFeS equal to 0.5 and 0.6, as indicated. Yellow circles are measurements of olivine-hosted melt inclusions from older eruptions of Piton de la Fournaise volcano (Bureau et al. 1998a, b). b Plot of S concentrations and Fe3+/∑Fe ratios. Small black diamonds are measured values, uncorrected for the effects of post-entrapment olivine crystallization on the melt inclusion wall. Pink diamonds are the compositions after correction for post-entrapment crystallization (PEC) of olivine on the melt inclusion wall. Thin black lines connect the pre-PEC and post-PEC compositions of the same inclusions. Thick black lines show the proposed effect of Fe-S redox coupling during quench as a function of the S content of a melt at ΔQFM ~ 0 and ΔQFM-0.6 according to Nash et al. (2019)
Fe3+/∑Fe ratios
The Fe3+/∑Fe ratios of glass in nine melt inclusions range from 0.094 to 0.162, a range greater than expected based only on the reproducibility of XANES measurements (± 0.01, e.g., Cottrell and Kelley 2009). The Fe3+/∑Fe ratios of Reunion melt inclusions are uncorrelated with S contents (Figs. 5b, 6b), in contrast with previous XANES measurements of Fe3+/∑Fe and S in OIB submarine glasses (see Sect. 1 above, and Brounce et al. 2017; Hartley et al. 2017; Helz et al. 2017; Moussallam et al. 2014, 2016, 2019; Shorttle et al. 2015; Novella et al. 2020). The Reunion glasses are also lower in Fe3+/∑Fe value than XANES-based measurements of other undegassed OIB glasses (Fig. 1a; 0.17–0.40 Fe3+/∑Fe). There is, however, a weak correlation between Fe3+/∑Fe and H2O contents for Reunion glasses (Fig. 6c) and we consider three processes that might account for this correlation and the observed Fe3+/∑Fe range more generally.
a H2O and S concentrations of olivine-hosted melt inclusions and glassy embayments. The solid and dashed curves are D-COMPRESS (Burgisser et al. 2015) model trajectories of the expected change in H2O and S during degassing in a closed (solid black curves) and open (black dashed curves) system. The stars mark the starting composition of open and closed system trajectories. Tick marks show the progression of pressure in bars. b Plot of Fe3+/∑Fe ratios and S concentrations of olivine-hosted melt inclusions, with the same degassing models as in panel a. c Fe3+/∑Fe ratios and H2O concentrations in olivine-hosted melt inclusions. The same degassing trajectories from panels a and b are shown. The thick pink curve is the modeled effect of diffusive re-equilibration of H2O and Fe3+/∑Fe according to Bucholz et al. (2013), with tick marks indicating time in hours. Approximate fO2 relative to QFM is shown on the left hand y-axis, calculated at fixed major element composition. Bolded pink diamond has the lowest Fe3+/∑Fe measured in this study, see text
Degassing
Degassing of S-rich gases is known to lead to a decrease in the Fe3+/∑Fe ratio of residual liquid under most conditions (Anderson and Wright 1972; Brounce et al. 2017; Burgisser and Scaillet, 2007; Candela 1986; Carmichael and Ghiorso 1986; Gaillard 2011, 2015; Hartley et al. 2017; Helz et al. 2017; Kelley and Cottrell 2012; Metrich et al. 2009; Moussallam et al. 2014, 2016, 2019; Shorttle et al. 2015). One possible explanation of our findings for Reunion glasses is that S degassing prior to or synchronous with inclusion entrapment (or even afterwards by degassing into vapor bubbles in the inclusions) generated the Fe3+/∑Fe variations we have observed. Although the inclusions we have studied do not appear to have undergone significant amounts of S loss by degassing (e.g., the lowest concentrations are in excess of 850 ppm S) and we observe no strong relationship between S and Fe3+/∑Fe (Figs. 5b, 6b), they do display a range in S contents (~ 850–1250 ppm). Additionally, melt inclusions from prior eruptions at Piton de la Fournaise extend the range of observed S contents significantly (~ 550–1760 ppm; Bureau et al. 1998a; Fig. 5a). Even though the inclusions studied here have Fe3+/∑Fe ratios that are uncorrelated with S content (Fig. 6b), we can estimate the expected range in Fe3+/∑Fe ratio that would arise by degassing alone using the D-COMPRESS model of Burgisser et al. (2015). According to this model, degassing to a final pressure of 1 bar of an initial melt with Fe3+/∑Fe = 0.16, 0.67 wt% H2O, 1200 ppm S, and 200 ppm CO2 (i.e., values consistent with this study and CO2 reported by Famin et al. 2009, both studies of melt inclusions from the same eruption) results in a decrease of Fe3+/∑Fe ratio to 0.12. However, if the predictions of the D-COMPRESS model are accurate (and it is possible that it provides a minimum estimate of the impact of S degassing on redox, because it assumes that oxidized S is speciated in the melt as SO2 rather than SO42−), neither closed nor open system degassing describes well the observed change in Fe3+/∑Fe with decreasing H2O contents (black solid and dashed curves, Fig. 6c) or the lack of a correlation between S content and Fe3+/∑Fe ratio (Fig. 6b) in the melt inclusion suite measured here. We thus conclude that although degassing may have influenced the melt inclusions we have studied, to the degree that the D-COMPRESS model accurately captures the effects of degassing on depressurization, such a process cannot simply explain the observed variations in Fe3+/∑Fe (but see Sect. 4.2.3 for an alternate degassing process that for the most part matches our observations).
Previous studies of melt inclusions from older eruptions of Piton de la Fournaise have measured H2O contents as high as 1.1 wt% and S contents as high as 1760 ppm S (Bureau et al. 1998a, b; Fig. 5a, 6). Additionally, measurements of fluid inclusions indicate very high CO2 contents of deeply stored magmas at Piton de la Fournaise (e.g., > 1 wt% CO2; Boudoire et al. 2018). This raises the possibility that the liquids trapped in the melt inclusions studied here evolved by degassing prior to entrapment from liquids that contained higher initial concentrations of CO2, H2O and S than observed by us in any of the melt inclusions we have studied. Using D-COMPRESS, we have estimated the range of possible initial values of Fe3+/∑Fe ratios that would be required to explain partially degassed compositions equivalent to melt inclusions studied here (gray star and connected curves, Fig. 6) and conclude that undegassed magmas with 1.1 wt% H2O, 200 ppm CO2, and 1700 ppm S could have Fe3+/∑Fe ratios no higher than 0.15–0.17 to degas to a Fe3+/∑Fe ratio of 0.12 and ~ 700 ppm S (gray star and associated black curves, Fig. 6b, c).
Temperature dependence of homogeneous equilibrium between Fe and S in melt
Another possibility that might in principle account for the range in observed Fe3+/∑Fe ratios in the centers of Reunion melt inclusions is that this ratio increases during cooling and quenching of the melt inclusion to glass, because the electron transfer reaction 8Fe2+ + S6+ = 8Fe3+ + S2− is driven to the right with decreasing temperature (Nash et al. 2019). A key prediction of this hypothesis is that the higher the S6+ content of the high-temperature melt, the more effective this mechanism is at increasing Fe3+/∑Fe ratio for a given FeO* and initial Fe3+/∑Fe. Assuming an initial fO2 of QFM-0.6 (calculated Fe3+/∑Fe = 0.120 for a glass with the major element composition of Reunion-3 from Kress and Carmichael 1991), 10 wt.% FeO*, total S contents ranging from 850 to 1250 ppm, and that cooling begins from 1160 °C (the estimated liquidus temperature of the melt in the center of a typical melt inclusion according to the MgO thermometer of Helz and Thornber 1987), we calculate that the Fe3+/∑Fe ratio would increase on quenching from 0.120 to 0.122 at 850 ppm S and from 0.120 to 0.126 at 1250 ppm S (Fig. 5b). This range of quenched Fe3+/∑Fe ratios is significantly smaller than the range observed in the Reunion melt inclusions. This—and the fact that there is no observed correlation between total S content and Fe3+/∑Fe ratio for these inclusions (Fig. 5b)—argue against this mechanism contributing significantly to the observed range of Fe3+/∑Fe ratio.
Post-entrapment diffusion through the enclosing olivine
The final possibility that we consider is that the variation in measured Fe3+/∑Fe ratios of the Reunion melt inclusions reflects post-entrapment diffusion of metal vacancies across the olivine host in response to a difference in fO2 between the melt inclusion and the host magma. It has been shown that H2O contents of olivine-hosted melt inclusions can reequilibrate with the surrounding magma by diffusion of H+ across the olivine in ~ 10 s of hours and that metal vacancies in the host olivine move at similar rates (Bucholz et al. 2013; Gaetani et al. 2012). Consequently, suites of variably re-equilibrated melt inclusions could record a range of Fe3+/∑Fe ratios and H2O concentrations due to varying extents of H2O and metal vacancy exchange across the olivine after entrapment (Bucholz et al. 2013; Gaetani et al. 2012). For example, suppose that the Reunion melt inclusions were trapped at pressures higher than those at which significant H2O and/or S degassing occurs; this would be the case if the melt inclusions were trapped at ~ 800 bars, the depth of magma storage of Reunion picritic magmas (Famin et al. 2009), since this is higher than the 330 bars at which, according to D-COMPRESS, a model undegassed Reunion liquid begins to degas significant quantities of H2O. The magma containing the olivine-hosted melt inclusions could then have ascended rapidly to a lower, near-surface pressure, at which point the liquid fraction of the magma would have degassed most of its H2O and S, resulting in a decrease in fO2 from ΔQFM = 0 to – 0.7. In contrast, a melt inclusion would not have degassed on ascent nearly as extensively into vapor bubbles as would the liquid enclosing its olivine host, because the inclusion would have experienced a significantly smaller decrease in pressure on ascent as it maintained an approximately constant volume. Such a situation would give rise to a low H2O, S, and fO2 liquid outside the olivine and a higher H2O, S, and fO2 liquid inside the melt inclusion. Because H2O, H-bearing defects, and metal vacancies can diffuse across the host olivine, total H2O concentration and fO2 in the melt inclusions can change on relatively short time scales, ultimately taking on values near those of the degassed enclosing liquid. In contrast, total S concentrations in the melt inclusions would be unchanged assuming S cannot be transported across the host olivine (Bucholz et al. 2013; Gaetani et al. 2012; Newcombe et al. 2014; Rowe et al. 2007; Saper and Stolper 2020).
The range in H2O contents and fO2 recorded by Reunion melt inclusions might thus reflect their variable extents of transfer of H-bearing species and metal vacancies across the olivine host, while the S contents of the inclusions would still largely reflect the undegassed magma originally trapped in the melt inclusions. We simulated this process and the expected changes in H2O and Fe3+/∑Fe in an initially hydrous and oxidized melt inclusion that is communicating diffusively with a relatively dry and reduced external magma using the numerical model of Bucholz et al. (2013). We assumed a 150 μm diameter spherical melt inclusion enclosed in the center of a 1 mm diameter olivine at 1100 °C; we also assumed that the melt inclusion initially contained 0.67 wt% H2O at QFM (Fe3+/∑Fe ~ 0.16) and that the host melt degassed from this initial composition to 1 bar, resulting in 0.05 wt% H2O and an fO2 of QFM-0.7 according to D-COMPRESS (equivalent to Fe3+/∑Fe ~ 0.120). Using the model from Bucholz et al. (2013), shown by the pink curve in Fig. 6c, a melt inclusion would approach equilibrium with the degassed external melt with 0.05 wt% H2O in ≲ 200 h, by which point the H2O in the melt inclusion would have decreased to 0.05 wt% and the Fe3+/∑Fe has decreased to ~ 0.125 (i.e., to the values of the external melt). The modelled concentration of metal vacancies across the olivine grain becomes nearly homogeneous after ~ 48 h, at which point the fO2 of the modelled melt inclusion is essentially equal to that of the external melt and little further change in fO2 (and Fe3+/∑Fe) in the melt inclusion occurs. At this time, the modelled olivine still has a gradient in H+, and so the H2O content of the modelled inclusion continues to decrease at roughly constant fO2; at 200 h, the model H2O content has changed 91% of the way towards the final value of 0.05 wt%.
With the exception of the data point with the lowest Fe3+/∑Fe data point (Reunion-8, shown as the pink diamond outlined in black in Fig. 6c), this diffusion model describes the melt inclusion measurements reasonably well, and most importantly, better than the simple degassing models described in Sect. 4.2.1 based on D-COMPRESS (shown in Fig. 6c as black solid and dashed curves). Based on the observations of Bucholz et al. (2013) and Gaetani et al. (2012) that 1H diffuses more rapidly through olivine hosts than 2H in experimentally dehydrated olivine-hosted melt inclusions, a prediction of this explanation for the observed correlation between Fe3+/∑Fe and dissolved H2O contents of the Reunion melt inclusions is these inclusions will also record a range in D/H ratios that vary as a function of progressive diffusive H2O loss from the inclusions.
As mentioned above, one melt inclusion with 0.42 wt% H2O (Reunion-8, shown as the pink diamond outlined in black in Figs. 4, 5, 6), has the lowest measured Fe3+/∑Fe (0.094). This sample is indistinguishable from the other inclusions included in this study in its major or volatile element composition, and it is unremarkable petrographically. Although it is always possible that some amount of olivine was included in the analytical volume, skewing the pre-edge spectral features to result in lower calculated Fe3+/∑Fe ratios than glass measurements alone, there is no spectral evidence for such “olivine contamination” elsewhere in the XANES spectrum (see Brounce et al. 2014). Otherwise, we have no testable explanation for this anomalous data point, although it could reflect variations among inclusions in the initial Fe3+/∑Fe of the trapped liquid or equilibration with a more reduced host liquid such that the plateau in the modeled curve in Fig. 6c would be at a lower Fe3+/∑Fe ratio.
In the context of the model described in this subsection and illustrated in Fig. 6c, the samples with the highest H2O and Fe3+/∑Fe ratios (Reunion-4, Reunion-10, and Reunion-15) would be most representative of the fO2 of the least degassed and/or the least re-equilibrated Reunion liquids sampled in melt inclusions. These samples have average Fe3+/∑Fe = 0.158 ± 0.005, H2O = 0.69 ± 0.02, and S = 1090 ± 110 ppm (Table 1), corresponding to an fO2 of ΔQFM ≈ 0, similar to the summary of previous estimates of fO2 for Reunion island lavas shown in Fig. 1b (which shows our data, that of Gaborieau et al. 2020, and previous data summarized by Pichavant et al. 2016). It is also similar to the average fO2 reported for MORB in the Indian ocean basin (Birner et al. 2018; Zhang et al. 2018). We emphasize, however, that we cannot rule out that even the liquids trapped in these three inclusions have lost some CO2, H2O and S prior to entrapment. Although we have no evidence that this occurred, we do note again the higher H2O and S contents of the samples of Bureau et al. 1998a, b from an earlier eruption (Figs. 5a, 6a) and inferences from fluid inclusions of elevated CO2 magmas stored at depth at Piton de la Fournaise. However, modeled degassing trajectories from such a volatile-rich magma show that the effect on Fe3+/∑Fe (and thus on fO2) is small—i.e., to reproduce the melt inclusions with Fe3+/∑Fe ≈ 0.158 with the volatile contents observed here, the undegassed magma could not have had an fO2 not higher than ΔQFM ≈ + 0.1.
Oxygen fugacities of Reunion Island and Indian Ocean MORB primary magmas
We present three approaches to estimating primary melt compositions (i.e., compositions immediately after segregation of primitive melt(s) from mantle residues and prior to crystallization-differentiation and degassing) based on our measurements and estimates (described in the following text) of the possible range of amounts of crystal fractionation prior to melt entrapment in olivine.
We first incrementally added equilibrium liquidus olivine back to the compositions of Reunion-4, Reunion-10, Reunion-15 (see Sect. 4.2.3) until they reached equilibrium with Fo91 olivine (consistent with the estimated olivine in the Reunion residual mantle source; Sobolev and Nikogosian 1994). Calculation of olivine addition was done as described in Sect. 4.1 for the post-entrapment crystallization correction. This required 24–31 wt% olivine addition and produces model melt compositions with 15.0–16.9 wt% MgO and 8.6–9.3 wt% CaO. The model estimate for CaO content is lower than expected for batch melts or accumulated fractional melts of a fertile or slightly depleted peridotitic source, suggesting that crystallization of high-Ca pyroxene in addition to olivine occurred prior to melt inclusion entrapment; this inference is consistent with prior studies of the liquid lines of descent of Reunion magmas (e.g., Albarede et al. 1997; Famin et al. 2009). The model primary melts reconstructed in this way have ΔQFM = -0.4 (calculated at 1 atm, 1150 °C); this fO2 is ~ 0.4 orders of magnitude lower than those calculated from measured Fe3+/∑Fe. The representative pressures and temperatures of melting independently estimated for Reunion lavas are ~ 3 GPa and 1400 °C (Albarède et al. 1997; Albarède and Tamagnan 1988; Sobolev and Nikogosian 1994). The effect of increasing temperature from 1150 °C to 1400 °C on the calculation of fO2 of a silicate magma relative to the QFM buffer is small (< 0.02 log units at 3 GPa; Brounce et al. 2017; Kress and Carmichael, 1991). However, the effect of increasing pressure from 1 atm to 3 GPa increases the fO2 of the silicate liquid by ~ 0.4 orders of magnitude relative to the QFM buffer at 1400 °C. The net result of the olivine addition calculations and projection to the high pressures and temperatures of melting nearly cancel one another, yielding an estimate of the fO2 of a Reunion primary melt as it separated from its source of ΔQFM ≈ 0 ± 0.1 (the average and standard deviation of the three values calculated for Reunion-4, Reunion-10, and Reunion-15; Fig. 7; Table 1).
Plot of the Fe3+/∑Fe ratios of “most parental” magmas (see Fig. 1) versus DSr−Nd−Pb of Jackson et al. (2020), an index for the extent of the shift away from geochemically depleted mantle with respect to Sr, Nd, and Pb isotopic compositions. The Fe3+/∑Fe ratios of “most parental” magmas at each location are taken from the literature (see Fig. 1), Reunion constraint (this study) described in the main text. Also shown are the calculated DSr−Nd−Pb of melts of DMM, C/FOZO/PREMA, EM1, EM2, and HIMU from Jackson et al. (2020), with various possible Fe3+/∑Fe ratios. The Fe3+/∑Fe ratio of the FOZO component is set equal to that calculated in this study, and MORB is set equal to that of Zhang et al. (2018). The Fe3+/∑Fe ratios of the blue, orange, and gray boxes (0.5) connected to MORB by solid curves is the value measured for the ODP Site 801 Super Composite powder (Brounce et al. 2019), meant to represent the Fe3+/∑Fe of bulk subducting altered oceanic crust and seafloor sediments. The endmembers connected to MORB by dashed curves have Fe3+/∑Fe of 0.7, illustrating the impact of mixing more oxidized melts on the mixing curve trajectories. Isotopic data for each island from Stracke et al. (2005), Jackson et al. (2020), and Sims et al. (2008)
We made a second estimate by adding olivine and clinopyroxene back to the composition of each of the same three melt inclusions until equilibrium with Fo91 olivine was achieved. This calculation was implemented using Petrolog3 (Danyushevsky and Plechov 2011), the mineral-melt models of Roeder and Emslie (1970) for olivine and Langmuir et al. (1992) for high-Ca clinopyroxene, and assuming that Fe3+ is perfectly incomaptible for both olivine and clinopyroxene. Equilibrium olivine and clinopyroxene were added to the compositions of Reunion-4, Reunion-10, and Reunion-15 in proportions dictated by the mineral-melt models cited above until a melt composition in equilibrium with Fo91 olivine was obtained. This required 20–23% pyroxene and 9–12% olivine addition using the selected melt models, and produced melt compositions with 13.1–14.7 wt% MgO and 12–12.2 wt% CaO, which are consistent with the range in MgO and CaO possible from accumulated fractional melts of a peridotitic source mantle (Herzberg and Asimow 2008). This calculation produces primary melt compositions in equilibrium with Fo91 olivine with average ΔQFM = – 0.2 ± 0.1 (corrected to 3 GPa, 1400 °C, as described for the preceding model calculation; Table 1). Note that using a non-zero value for DFe3+cpx/melt (e.g., ~ 0.5; Sorbadere et al. 2018) would yield a primary magma with slightly higher fO2.
The final calculation was based on the PriMelt2 calculator (Herzberg and Asimow 2008), which is similar to the first calculation presented above except that it simultaneously calculates the composition of accumulated fractional melts of a peridotitic mantle source and thus returns a parental melt composition that is consistent with having been produced by melting of a peridotitic mantle source. This yields modeled primary melt compositions for the Reunion-4, Reunion-10, and Reunion-15 melt inclusion compositions in equilibrium with Fo91.5–Fo91.9 olivine at 1370–1430 °C and 3.0 GPa, and ΔQFM ≈ 0 ± 0.1 at the model temperatures and pressures (Table 1). As pointed out above and by Herzberg and Asimow (2008), the PriMelt2 calculations indicate that these reconstructed primary melts have CaO concentrations that are too low to have segregated from high-Ca pyroxene-bearing residues and, therefore, suggest that significant pyroxene fractionation was involved in producing the melts that were trapped as inclusions analyzed in this study.
These calculations show the insensitivity of estimates of the fO2 values of reasonable primary melts to the precise model choice of how to correct for crystal fractionation. Taken together, the results of these calculations suggest fO2 values for the primary liquid of the 2005 Piton de la Fournaise eruption of ΔQFM = – 0.2 to 0.0 at the point of its separation from the solid residue in its source at approximately 3 GPa, 1400 °C. Assuming that the effect of partial melting on the fO2 of the melt and mantle residue is small up to ~ 25% melting of peridotite (e.g., Sorbadere et al. 2018; Davis and Cottrell 2018); that the melt and residue were in equilibrium and thus reflect the same fO2 up to the point of melt segregation; and that we can approximate melting with a single stage of melt extraction), we take this as a reasonable estimate for the fO2 of the mantle sources of Piton de la Fournaise magmas. Estimates of the fO2 of the Indian upper mantle at the pressures and temperatures of primary melt generation (1.25 GPa, 1320 °C; taken directly from Birner et al. 2018), are similar to those for the primitive, undegassed Reunion melt inclusions, ranging from ΔQFM = – 0.5 to + 0.1, with an ocean basin average value of ΔQFM = – 0.1 ± 0.1 (Fig. 7).
The estimate of the fO2 of the mantle source of the 2005 Piton de la Fournaise primary liquid presented here (and by other studies of Reunion Island lavas; see Fig. 1b and Pichavant 2016; Gaborieau et al. 2020; Peters et al. 2019) is significantly lower (i.e., outside of analytical uncertainty of ± 0.01 Fe3+/ΣFe; ± 0.25 log units fO2) than recently published estimates of the fO2 of the mantle sources of other ocean island lavas based on similar analytical approaches and primary melt reconstructions (Figs. 1a, 7). Moreover, the fO2s inferred for mantle sources of lavas from Hawaii (ΔQFM = + 0.5 to + 0.6; Brounce et al. 2017; Helz et al. 2017; Moussallam et al. 2016), Iceland (ΔQFM = + 0.4 to + 0.5; Hartley et al. 2017; Shorttle et al. 2015; Novella et al. 2020), Mount Erebus (ΔQFM = + 1.7; Moussallam et al. 2014) and the Canaries (ΔQFM > + 2; Moussallam et al. 2019) are all significantly higher than the fO2 estimates of the upper mantle that feeds the mid-ocean ridge system in their respective ocean basins (Moussallam et al. 2019). In contrast, the estimate of the fO2 relative to QFM of the Reunion source presented here (ΔQFM ~ – 0.2 to 0) cannot be distinguished from that of the Indian upper mantle (ΔQFM = – 0.5 to + 0.1; Birner et al. 2018; Zhang et al. 2018). This is a key result of our study, demonstrating that not all OIB magmas record higher fO2s in their sources than ambient upper mantle as represented by the sources of MORBs from the same ocean basin, contrary to the conclusion of Moussallam et al. (2019).
The Reunion samples we have studied have Pb isotope ratios resembling the so-called DUPAL anomaly and elevated 87Sr/86Sr relative to DMM (Bosch et al. 2008). If these isotopic characteristics are due to the presence of oxidized recycled materials in the sources of Reunion magmas, they must be present in proportions too small to elevate significantly the fO2 of Reunion magmas above that of the DMM reservoir sampled by Indian Ocean MORB. Furthermore, as much as 60–90% of the Reunion mantle source has been suggested to consist of the C/FOZO/PREMA component (Bosch et al. 2008). To the extent that this isotopically defined mantle component of many OIB lavas reflects a specific mantle lithology with a well-defined origin and history, our results suggest that this component has an fO2 of ΔQFM ≈ 0 at ~ 3 GPa and 1350 °C, not significantly different from that of DMM at ~ 1 GPa and 1250 °C (i.e., the dominant upper mantle reservoir). If this is correct, it would argue against significant contributions of oceanic crust recycled in the last 400–800 my to the C/FOZO/PREMA mantle component, because more recently generated oceanic crust is inferred to have become significantly oxidized during their time at or near Earth’s surface (e.g., Lecuyer and Ricard, 1999; Stolper and Keller 2018).
There are many factors that could influence the fO2 of a partial melt of the mantle including: the major element composition of the source, which influences the mineralogy and phase equilbria under both subsolidus and supersolidus conditions as functions of P and T and could lead to variations of at least one order of magnitude in fO2 (e.g., Jennings and Holland, 2015; Stolper et al. 2020); the concentrations and oxidation states of heterovalent elements in the source, including not only Fe, but also H, C, S, and more (e.g., Frost and McCammon 2008 and references therein); and the flow of fluids through the source (e.g., Frost and McCammon 2008 and references therein). Given these and other possible factors that could influence the fO2 levels of the mantle sources of magmas, it is difficult at this time to decode the significance of the essentially identical fO2 levels of magmas from the C/FOZO/PREMA-rich Reunion plume source (as defined by this study and previous studies of different lavas from Reunion island, Fig. 1b) and MORB magmas from the global upper mantle DMM source. Given the sensitivity of fO2 to so many factors, this close correspondence is unlikely to be a coincidence, although this cannot be ruled out. One possible explanation for this similarity is that melt extraction ± addition processes similar to those acting on mantle corresponding to the bulk silicate earth that resulted in the chemical composition of the DMM source—including its fO2—also had a significant role in the generation of the apparently widespread C/FOZO/PREMA source early in Earth history. In this scenario, a simple explanation for the differences between the Reunion source and the sources of OIBs with mantle sources that record higher fO2s reflects the larger contributions to the sources of these other OIBS of higher fO2, recycled EMI, EMII and HIMU source components. If this is correct, it suggests that the various shared characteristics of Reunion and other, more oxidized OIB magmas (i.e., at Hawaii, Iceland, Canary Islands, Erebus)—such as elevated pressures and temperatures of melting and larger amounts of crystal fractionation at higher average pressures than typical MORBs—do not uniquely set the fO2 of OIB magmas since otherwise, Reunion magmas would be expected to be as oxidized as Hawaiian, Icelandic, Canary Island, and Erebus magmas.
Finally, we point out that there is substantial isotopic heterogeneity of the C/FOZO/PREMA component (as there is in DMM, EM1, EM2, HIMU components as well, e.g., Stracke et al. 2005) globally and it is possible that this extends to fO2 as well. At this time there are not to our knowledge other constraints on the fO2 of C/FOZO/PREMA component. Additional studies of relatively undegassed magma compositions (including fO2) at locations that sample C/FOZO/PREMA without significant additions of EM1, EM2, and HIMU components (which may be very oxidized, see Sect. 4.4.) will be necessary to assess this.
Heterogeniety in mantle source fO2 and radiogenic isotope ratios
We have emphasized that the elevated Fe3+/ΣFe ratios and inferred fO2 levels of relatively undegassed glasses from Hawaii, Iceland, the Canaries, and Mount Erebus relative to MORB and Reunion magmas have been suggested as being related to the presence in their mantle sources of isotopically enriched EM1 and HIMU components that are oxidized relative to DMM (Brounce et al. 2017; Hartley et al. 2017; Moussallam et al. 2014, 2016, 2019; Shorttle et al. 2015; Novella et al. 2020). Although, as discussed above, many factors can influence the fO2 levels of the mantle sources of basaltic magmas, in this final section, we extend the modeling by Jackson et al. (2020) of source mixing (constrained by variations in radiogenic isotope ratios in OIBs) to include their Fe3+/ΣFe ratios. The goal of this modeling is to evaluate semi-quantitatively whether the differences in fO2 estimates for the Hawaiian and Icelandic sources on one hand (ΔQFM ~ + 0.4 to + 0.6; Brounce et al. 2017; Hartley et al. 2017; Moussallam et al. 2016; Shorttle et al. 2015; Novella et al. 2020) and for Mount Erebus and the Canary Islands on the other (ΔQFM ≳ + 2; Moussallam 2014, 2019) are consistent with the proportions of mantle end members in their sources based on radiogenic isotopic ratios. For example, one simple model would be that EM1, EM2, and HIMU end members have similar major element compositions (including Fe3+/ΣFe ratios), in which case they would typically have similar fO2 at a given P and T. If these end members were mixed with other source components (e.g., DMM, EM1, EM2, HIMU), again with major element compositions of the enriched components assumed to be similar, then if Hawaiian and Icelandic mantle sources contained lower proportions of recycled surface materials relative to the sources of Mount Erebus/Canary Islands, this could explain the lower Fe3+/ΣFe ratios (and lower inferred fO2s; Fig. 7) of Hawaiian/Icelandic basaltic glasses.
We have evaluated this type of mixing model semi-quantitatively using the model of Jackson et al. (2020) to constrain the proportions of the isotopically defined (and distinguishable) end members in the sources of the OIBs; then, assuming values for the Fe3+/ΣFe ratios of the isotopically defined end members, we evaluate whether the isotopic and Fe3+/ΣFe ratios can be simulatenously satisified for the Hawaiian, Icelandic, Canary Islands, and Erebus OIBs given the proportions of the end members in their sources based on the isotopc ratios of the OIBs from Jackson et al. (2020). To simplify this exercise, we assume that all melts of all end members have 11 wt% FeO* and that melts of EM1, EM2, and HIMU all have the same Fe3+/ΣFe ratio, corresponding to that of altered oceanic crust, as represented by the “Super Composite” of seafloor sediments and altered oceanic crust recovered from the Pacific ocean crust at ODP Site 801 (Fe3+/ΣFe ~ 0.5, see Brounce et al. 2019). We also assume that a partial melt of DMM has Fe3+/ΣFe = 0.12 (Cottrell and Kelley 2011; Zhang et al. 2018). We then mix the partial melt of DMM and melts of the enriched source components in various proportions between 0 and 1 (i.e., duplicating the approach of Jackson et al. 2020) to generate the solid blue, orange, and gray mixing curves shown in Fig. 7 for mixtures of DMM with EM1, HIMU, and EM2, respectively; the DSr-Nd-Pb parameter shown on the x-axis is defined by Jackson et al. (2020) and is calculated for each mixing ratio (and for each of the basalt samples shown as colored dots in Fig. 7) using the endmember Sr and Pb concentrations and 87Sr/86Sr and 206Pb/204Pb ratios of melts of EM1, HIMU, and DMM estimated by Jackson et al. (2020).
For both Iceland and Hawaii, the estimated proportions of EM1 in their sources based on their radiogenic isotope ratios (DSr-Nd-Pb = 0.35 ± 0.07, DSr-Nd-Pb = 0.33 ± 0.04, respectively) are about 5% based on their radiogenic isotope ratios based on Jackson et al. (2020); this estimate is consistent with other estimates of the proportion of enriched components for Hawaiian magmas on the basis of other isotope systems and trace elements of 4–20% (Eiler et al. 1996; Hauri et al. 1996; Sobolev et al. 2007). The proportions of the EM1 endmember in the source required to match the Fe3+/ΣFe ratios of relatively undegassed Hawaiian and Icelandic glasses given the parameters described above are ~ 15% EM1 (based on the tick marks on the blue curve in Fig. 7). This overlaps the upper end of the range of estimated EM1 proportions based on radiogenic isotopes and demonstrates that the amount of EM1 in Hawaiian and Icelandic sources based on Fe3+/ΣFe and isotopic ratios are compatible.
For the Canary Islands, the estimated proportions of HIMU in their sources are about 30% from their radiogenic isotope ratios (DSr-Nd-Pb = 0.55 ± 0.06) based on Jackson et al. (2020); as for Hawaii and Iceland, this is again broadly consistent with previous esimates of 10–35% HIMU component in the sources of Canary Island magmas (Widom et al. 1999; Day et al. 2010). The proportions of the HIMU end member in the source required to match the Fe3+/ΣFe ratios of relatively undegassed Canary Island glasses are ~ 40% (based on the tick marks on the solid orange curve in Fig. 7), again consistent with the range of estimated HIMU proportions based on radiogenic isotopes. Finally, for Mount Erebus, the estimated proportions of HIMU in the sources from radiogenic isotopes is ~ 40% (DSr-Nd-Pb = 0.63 ± 0.03) based on Jackson et al. (2020) and 35–95% based on Phillips et al. (2018). The proportions of the HIMU end member in the source required to match the Fe3+/ΣFe ratios of relatively undegassed Erebus glasses are 55% (based on the tick marks on the solid orange curve in Fig. 7), consistent with the range of estimated HIMU proportions based on radiogenic isotopes. Given the unrealistically simple assumptions of this mixing model, we are encouraged by the overall level of agreement between the higher amounts of HIMU in their sources implied by the isotopes of the Canary Islands and Erebus samples relative to amounts of the EM1 components implied for the sources of the Hawaii and Iceland samples and the success of the mixing model in explaining simultaneously the isotopic ratios and Fe3+/ΣFe ratios presented here for these four OIB. We also considered the possibility that the EM1, EM2, and HIMU end members have a higher Fe3+/ΣFe ratio. If we assume that partial melts of the end-member source components have Fe3+/ΣFe ~ 0.7, the fit of these simple mixing models (shown by the dashed curves in Fig. 7) to the radiogenic isotopic compositions and estimates of the Fe3+/ΣFe of the least degassed magmas at each location is improved, but there are so many uncertainties in the model in detail that we do not regard this improvement as significant. For example, including the likely higher melt productivity of the enriched components relative to DMM would produce the same sense of change introduced by assuming a higher Fe3+/ΣFe ratio in the enriched sources, and the assumptions about the total Fe contents of the partial melts and/or the source components would also have consequences.
Overall, however, although the mixing model coupling radiogenic isotopic ratios and Fe3+/ΣFe ratios depends on many simplifying assumptions and approximations that can be challenged, they demonstrate that the Fe3+/ΣFe ratios in OIB glasses from Hawaii, Iceland, the Canary Islands, and Mt. Erebus can be explained semi-quantitatively by a simple mixing model using proportions of DMM and EM1 or HIMU components in their sources based on the radiogenic isotope ratios of basaltic rocks from these OIBs. It remains to be seen if glasses from other OIBs can be similarly explained, but this can be readily tested with measurements of the Fe3+/ΣFe of magmas whose isotopic compositions reflect significant and variable contributions from these same endmembers—such as St. Helena, Tubaii, Rurutu, Mangaia, and Rimatara islands for HIMU (Stracke et al. 2003) and Tristan da Cunha, Pitcairn, and Kerguelen islands for EM1 (Stracke et al. 2003). Additionally, the model predicts the behavior of the EM2 component, represented by Society, Samoa, and Marquesas islands, for example (Stracke et al. 2003), for which Fe3+/ΣFe ratios are currently unavailable.
Conclusions
Olivine-hosted melt inclusions from the 2005 Piton de la Fournaise eruption at Reunion Island have Fe3+/ΣFe ratios that are indistinguishable from those of Indian Ocean MORB, in contrast to the recent suggestion that all ocean island basaltic magmas are more oxidized than MORB (e.g., Moussallam et al. 2019). The Fe3+/ΣFe ratios of these inclusions correlate weakly with measured H2O concentrations but not with S; this can be explained by incomplete diffusive reequilibration of Fe3+/ΣFe and H2O of the melt inclusions through the olivine hosts with a degassed and thus reduced external magma. The least degassed inclusions are used to reconstruct the fO2 of the primary magmas from which they were derived, at the mantle conditions under which they likely equilibrated with their mantle sources; this reconstruction suggests that the mantle sources of the 2005 Piton de la Fournaise eruption had fO2 levels corresponding to ΔQFM-0.1 ± 0.1 (also indistinguishable from reconstructed MORB primary melts). Because the mantle source of magmas at Piton de la Fournaise are thought to consist of ~ 60–90% of the C/FOZO/PREMA mantle component, this suggests the fO2 of this mantle component is essentially indistinguishable from that of DMM. This in turn may suggest that the igneous processes that led to the well-defined and similar fO2 of both the DMM and C/FOZO/PREMA reservoirs (e.g., melt extraction and/or addition relative to initially similar reservoirs) either did not result in major changes in fO2 relative to bulk silicate Earth and/or led to similar changes for both DMM and C/FOZO/PREMA. Although the C/FOZO/PREMA component present in the sources of Reunion magmas is not oxidized relative to DMM, the sources of other OIBs with significant contributions of EM1 and HIMU mantle components based on available radiogenic isotopic ratios are more oxidized than DMM based on available data. Simple semi-quantitative mixing models couple plausibly elevated Fe3+/ΣFe ratios of EM1, EM2, and HIMU components and their radiogenic isotopic compositions to variations in their contributions to the primary melts of OIB. The exact fO2 levels (and Fe3+/ΣFe ratios) of these reservoirs, (and that of EM2), and whether HIMU and EM1 are similarly oxidized (as suggested by Moussallam et al. 2019) or not, requires additional analyses of glasses from oceanic volcanos in which the EM1, EM2, and HIMU endmembers are thought to represent high fractions of their mantle sources.
References
Albarède F, Tamagnan V (1988) Modelling the recent geochemical evolution of the piton de la Fournaise volcano, Reunion Island, 1931–1986*. J Petrol 29:997–1030. https://doi.org/10.1093/petrology/29.5.997
Albarède F, Luais B, Fitton G, Semet M, Kaminski E, Upton BGJ, Bachèlery P, Cheminée J-L (1997) The geochemical regimes of piton de la Fournaise volcano (Réunion) during the last 530 000 years. J Petrol 38:171–201. https://doi.org/10.1093/petroj/38.2.171
Anderson AT, Wright TL (1972) Phenocrysts and glass inclusions and their bearing on oxidation and mixing of basaltic magmas, Kilauea Volcano, Hawaii. Am Min 57:188–216
Berry AJ, Stewart GA, O’Neill H, St C, Mallmann G, Mosselmans JFW (2018) A re-assessment of the oxidation state of iron in MORB glasses. Earth Planet Sci Lett 483:114–123. https://doi.org/10.1016/j.epsl.2017.11.032
Birner SK, Cottrell E, Warren JM, Kelley KA, Davis FA (2018) Peridotites and basalts reveal broad congruence between two independent records of mantle fO2 despite local redox heterogeneity. Earth Planet Sci Lett 494:172–189. https://doi.org/10.1016/j.epsl.2018.04.035
Bosch D, Blichert-Toft J, Moynier F, Nelson BK, Telouk P, Gillot P-Y, Albarède F (2008) Pb, Hf and Nd isotope compositions of the two Réunion volcanoes (Indian Ocean): A tale of two small-scale mantle “blobs”? Earth Planet. Sci Lett 265:748–765. https://doi.org/10.1016/j.epsl.2007.11.018
Boyet M, Doucelance R, Israel C, Bonnand P, Auclair D, Suchorksi K, Bosq C (2019) New constraints on the origin of EM-1 Component revealed by the measurement of the La-Ce isotope systematics in Gough Island lavas. Geochem Geosyst Geophys. https://doi.org/10.1029/2019GC008228
Borisov A, Behrens H, Holtz F (2018) Ferric/ferrous ratio in silicate melts: a new model for 1 atm data with special emphasis on the effects of melt composition. Contri Mineral Petrol 173(12):98. https://doi.org/10.1007/s00410-018-1524-8
Boudoire G, Rizzo AL, Di Muro A, Grass F, Liuzzo M (2018) Extensive CO2 degassing in the upper mantle beneath oceanic basaltic volcanoes: First insights from Piton de la Fournaise volcano (La Reunion Island). Geochimica et Cosmochimica Acta 235:376-401. https://doi.org/10.1016/j.gca.2018.06.004 .
Brounce MN, Kelley KA, Cottrell E (2014) Variations in Fe3+/∑Fe of mariana Arc Basalts and Mantle Wedge fO2. J Petrol 55:2513–2536. https://doi.org/10.1093/petrology/egu065
Brounce M, Stolper E, Eiler J (2017) Redox variations in Mauna Kea lavas, the oxygen fugacity of the Hawaiian plume, and the role of volcanic gases in Earth’s oxygenation. Proc Natl Acad Sci 114:8997. https://doi.org/10.1073/pnas.1619527114
Brounce M, Cottrell E, Kelley KA (2019) The redox budget of the Mariana subduction zone. Earth Planet Sci Lett 528:115859. https://doi.org/10.1016/j.epsl.2019.115859
Bucholz CE, Gaetani GA, Behn MD, Shimizu N (2013) Post-entrapment modification of volatiles and oxygen fugacity in olivine-hosted melt inclusions. Earth Planet Sci Lett 374:145–155. https://doi.org/10.1016/j.epsl.2013.05.033
Bureau H, Pineau F, Metrich N, Javoy M (1998a) A melt and fluid inclusion study of the gas phase at Piton de la Fournaise volcano (Réunion Island). Chem Geol 147:115–130
Bureau H, Metrich N, Semet MP (1998b) Magma-conduit interaction at Piton de la Fournaise (Réunion Island): a melt and fluid inclusion study. J Volc Geotherm Res 84:39–60
Burgisser A, Scaillet B (2007) Redox evolution of a degassing magma rising to the surface. Nature 445:194–197
Burgisser A, Alletti M, Scaillet B (2015) Simulating the behavior of volatiles belonging to the C-O–H–S system in silicate melts under magmatic conditions with the software D-Compress. Comput Geosci 79:1–14. https://doi.org/10.1016/j.cageo.2015.03.002
Candela PA (1986) The evolution of aqueous vapor from silicate melts: Effect on oxygen fugacity. Geochim Cosmochim Acta 50:1205–1211
Carmichael ISE, Ghiorso MS (1986) Oxidation-reduction relations in basic magma: a case for homogeneous equilibria. Earth Planet Sci Lett 78:200–210. https://doi.org/10.1016/0012-821X(86)90061-0
Castillo P (2015) The recycling of marine carbonates and sources of HIMU and FOZO ocean island basalts. Lithos 216–217:254–263. https://doi.org/10.1016/j.lithos.2014.12.005
Chauvel C, Hofmann AW, Vidal P (1992) himu-em: The French Polynesian connection. Earth Planet Sci Lett 110:99–119. https://doi.org/10.1016/0012-821X(92)90042-T
Cordier C, Delavault H, Chauvel C (2021) Geochemistry of the Society and Pitcairn-Gambier mantle plumes: What they share and do not share. Geochim Cosmochim Acta 306:362–384. https://doi.org/10.1016/j.gca.2021.04.014
Cottrell E, Kelley KA (2011) The oxidation state of Fe in MORB glasses and the oxygen fugacity of the upper mantle. Earth Planet Sci Lett 305:270–282. https://doi.org/10.1016/j.epsl.2011.03.014
Cottrell E, Kelley KA, Lanzirotti A, Fischer RA (2009) High-precision determination of iron oxidation state in silicate glasses using XANES. Chem Geol 268:167–179. https://doi.org/10.1016/j.chemgeo.2009.08.008
Danyuskevsky LV, Plechov P (2011) Petrolog3: integrated software for modeling crystallization processes. Geochem Geophys Geosyst 12:Q07021. https://doi.org/10.1029/2011GC03516
Davis FA, Cottrell E (2018) Experimental investigation of basalt and peridotite oxybarometers: Implications for spinel thermodynamic models and Fe3+ compatibility during generation of upper mantle melts. Am Mineral 103:1056–1067. https://doi.org/10.2138/am-2018-6280
Day JMD, Pearson DG, Macpherson CG, Lowry D, Carracedo JC (2010) Evidence for distinct proportions of subducted oceanic crust and lithosphere in HIMU-type mantle beneath El Hierro and La Palma, Canary Islands. Geochim Et Cosmochim Acta 74:6565–6589. https://doi.org/10.1016/j.gca.2010.08.021
Dupré B, Allègre CJ (1983) Pb–Sr isotope variation in Indian Ocean basalts and mixing phenomena. Nature 303:142–146. https://doi.org/10.1038/303142a0
Eiler JM, Farley KA, Valley JW, Hoffman AW, Stolper EM (1996) Oxygen isotope constraints on the sources of Hawaiian volcanism. Earth and Planet. Sci. Lett. 144:453–469. https://doi.org/10.1016/S0012-821X(96)00170-7
Famin V, Welsch B, Okumura S, Bachèlery P, Nakashima S (2009) Three differentiation stages of a single magma at Piton de la Fournaise volcano (Reunion hot spot). Geochem Geophys Geosystems. https://doi.org/10.1029/2008GC002015
Frost DJ, McCammon CA (2008) The Redox State of Earth’s Mantle. Annu Rev Earth Planet Sci 36:389–420. https://doi.org/10.1146/annurev.earth.36.031207.124322
Gaborieau M, Laubier M, Bolfan-Casanova N, McCammon CA, Vantelon D, Chumakov AI, Schiavi F, Neuville DR, Venugopal S (2020) Determination of Fe3+/∑Fe of olivine-hosted melt inclusions using Mossbauer and XANES spectroscopy. Chem Geol 547:119646. https://doi.org/10.1016/j.chemgeo.2020.119646
Gaetani GA, O’Leary JA, Shimizu N, Bucholz CE, Newville M (2012) Rapid reequilibration of H2O and oxygen fugacity in olivine-hosted melt inclusions. Geology 40:915–918. https://doi.org/10.1130/G32992.1
Gaillard F, Scaillet B, Arndt N (2011) Atmospheric oxygenation caused by a change in volcanic degassing pressure. Nature 478:229–232. https://doi.org/10.1038/nature10460
Gaillard F, Scaillet B, Pichavant M, Iacono-Marziano G (2015) The redox geodynamics linking basalts and their mantle sources through space and time. Chem. Geol. 418:217–233. https://doi.org/10.1016/j.chemgeo.2015.07.030
Garapic G, Jackson MG, Hauri EH, Hart SR, Farley KA, Blusztajn JS, Woodhead JD (2015) A radiogenic isotope (He-Sr-Nd-Pb-Os) study of lavas from Pitcarin hotspot: Implications for the origin of EM-1 (enriched mantle 1). Lithos 228–229:1–11. https://doi.org/10.1016/j.lithos.2015.04.010
Giuliani A, Jackson MG, Fitzpayne A, Dalton H (2021) Remnants of early Earth differentiation in the deepest mantle-derived lavas. PNAS. https://doi.org/10.1073/pnas.2015211118
Hanan BB, Graham DW (1996) Lead and helium isotope evidence from oceanic basalts for a common deep source of mantle plumes. Science 272:991. https://doi.org/10.1126/science.272.5264.991
Hart SR (1984) A large-scale isotope anomaly in the Southern Hemisphere mantle. Nature 309:753–757. https://doi.org/10.1038/309753a0
Hart SR, Hauri EH, Oschmann LA, Whitehead JA (1992) Mantle plumes and entrainment: isotopic evidence. Science 256:517. https://doi.org/10.1126/science.256.5056.517
Hartley ME, Shorttle O, Maclennan J, Moussallam Y, Edmonds M (2017) Olivine-hosted melt inclusions as an archive of redox heterogeneity in magmatic systems. Earth Planet Sci Lett 479:192–205. https://doi.org/10.1016/j.epsl.2017.09.029
Hauri EH, Whitehead JA, Hart SR (1994) Fluid dynamic and geochemical aspects of entrainment in mantle plumes. J Geophys Res 99:24275–24300
Hauri EH, Lassiter JC, DePaolo DJ (1996) Osmium isotope systematics of drilled lavas from Mauna Loa, Hawaii. J Geophys Res Solid Earth 101:11793–11806
Helz RT, Cottrell E, Brounce MN, Kelley KA (2017) Olivine-melt relationships and syneruptive redox variations in the 1959 eruption of Kīlauea Volcano as revealed by XANES. J Volcanol Geotherm Res 333–334:1–14. https://doi.org/10.1016/j.jvolgeores.2016.12.006
Helz RT, Thornber CR (1987) Geothermometry of Kilauea Iki lava lake, Hawaii. Bull Volc 49:651–668. https://doi.org/10.1007/BF01080357
Herzberg C, Asimow PD (2008) Petrology of some oceanic island basalts: PRIMELT2.XLS software for primary magma calculation. Geochem Geophys Geosystems. https://doi.org/10.1029/2008GC002057
Hofmann AW, White WM (1982) Mantle plumes from ancient oceanic crust. Earth Planet Sci Lett 57:421–436. https://doi.org/10.1016/0012-821X(82)90161-3
Jackson MG, Blichert-Toft J, Halldórsson SA, Mundl-Petermeier A, Bizimis M, Kurz MD, Price AA, Hardardóttir S, Willhite LN, Breddam K, Becker TW, Fischer R (2020) Ancient helium and tungsten isotopic signatures preserved in mantle domains least modified by crustal recycling. PNAS 117:30993–31001
Jayasuriya KD, O’Neill H St. C, Berry, AJ, Campbell SJ (2004) A Mössbauer study of the oxidation state of Fe in silicate melts. Am Mineral 89(11–12):1597–1609. https://doi.org/10.2138/am-2004-11-1203
Jenner FE, O’Neill H, St C (2012) Analysis of 60 elements in 616 ocean floor basaltic glasses. Geochem Geophys Geosystems. https://doi.org/10.1029/2011GC004009
Jennings ES, Holland TJB (2015) A simple thermodynamic model for melting of peridotite in the system NCFMASOCr. J Petrol 56:869–892
Kelley KA, Cottrell E (2009) Water and the oxidation state of subduction zone magmas. Science 325:605–607
Kelley KA, Cottrell E (2012) The influence of magmatic differentiation on the oxidation state of Fe in a basaltic arc magma. Earth Planet Sci Lett 329–330:109–121. https://doi.org/10.1016/j.epsl.2012.02.010
Langmuir CH, Klein EM, Plank T (1992) Petrological systematics of mid-ocean ridge basalts: constraints on melt generation beneath ocean ridges. Geophys Monogr Ser 71:183–280
Le Voyer M, Asimow PD, Mosenfelder JL, Guan Y, Wallace PJ, Schiano P, Stolper EM, Eiler JM (2014) Zonation of H2O and F concentrations around melt inclusions in olivines. J Petrol 55:685–707. https://doi.org/10.1093/petrology/egu003
Lecuyer C, Ricard Y (1999) Long-term fluxes and budget of ferric iron: implication for the redox states of the Earth’s mantle and lithosphere. Earth Plant Sci Lett 165:197–211. https://doi.org/10.1016/S0012-821X(98)00267-2
Metrich N, Berry AJ, O’Neill H, St C, Susini J (2009) The oxidation state of sulfur in synthetic and natural glasses determined by X-ray absorption spectroscopy. Geochim Cosmochim Acta 73:2382–2399. https://doi.org/10.1016/j.gca.2009.01.025
Moussallam Y, Oppenheimer C, Scaillet B, Gaillard F, Kyle P, Peters N, Hartley M, Berlo K, Donovan A (2014) Tracking the changing oxidation state of Erebus magmas, from mantle to surface, driven by magma ascent and degassing. Earth Planet Sci Lett 393:200–209. https://doi.org/10.1016/j.epsl.2014.02.055
Moussallam Y, Edmonds M, Scaillet B, Peters N, Gennaro E, Sides I, Oppenheimer C (2016) The impact of degassing on the oxidation state of basaltic magmas: A case study of Kīlauea volcano. Earth Planet Sci Lett 450:317–325. https://doi.org/10.1016/j.epsl.2016.06.031
Moussallam Y, Longpré M-A, McCammon C, Gomez-Ulla A, Rose-Koga EF, Scaillet B, Peters N, Gennaro E, Paris R, Oppenheimer C (2019) Mantle plumes are oxidised. Earth Planet Sci Lett 527:115798. https://doi.org/10.1016/j.epsl.2019.115798
Nash WM, Smythe DJ, Wood BJ (2019) Compositional and temperature effects on sulfur speciation and solubility in silicate melts. Earth Planet Sci Lett 507:187–198. https://doi.org/10.1016/j.epsl.2018.12.006
Newcombe ME, Fabbrizio A, Zhang Y, Ma C, Le Voyer M, Guan Y, Eiler JM, Saal AE, Stolper EM (2014) Chemical zonation in olivine-hosted melt inclusions. Contrib Mineral Petrol 168:1030. https://doi.org/10.1007/s00410-014-1030-6
Newcombe ME, Plank T, Barth A, Asimow PD, Hauri EH (2020) Water-in-olivine magma ascent chronometry: Every crystal is a clock. J Volc Geophys Res 398:106872. https://doi.org/10.1016/j.volgeores.2020.106872
Novella D, Maclennan J, Shorttle O, Prytulak J, Murton BJ (2020) A multi-proxy investigation of mantle oxygen fugacity along the Reykjanes Ridge. Earth Planet. Sci. Lett. 531:115973
O’Neill H, St C, Berry AJ, Mallmann G (2018) The oxidation state of iron in Mid-Ocean Ridge Basaltic (MORB) glasses: Implications for their petrogenesis and oxygen fugacities. Earth Planet Sci Lett 504:152–162. https://doi.org/10.1016/j.epsl.2018.10.002
O’Neill H, St C, Mavrogenes J (2002) The sulfide capacity and the sulfur content at sulfide saturation of silicate melts at 1400C and 1 bar. J Petrol 43:1049–1087. https://doi.org/10.1093/petrology/43.6.1049
Peters BJ, Shahar A, Carlson RW, Day JMD, Mock TD (2019) A sulfide perspective on iron isotope fractionation during ocean island basalt petrogenesis. Geochim Cosmochim Acta 245:59–78. https://doi.org/10.1016/j.gca.2018.10.015
Philipps EH, Sims KWW, Blichert-Toft J, Aster RC, Gaetani GA, Kyle PR, Wallace PJ, Rasmussen DJ (2018) The nature and evolution of mantle upwelling at Ross Island, Antarctica, with implications for the source of HIMU lavas. Earth Planet Sci Lett 498:38–53. https://doi.org/10.1016/j.epsl.2018.05.049
Pichavant M, Brugier Y, Di Muro A (2016) Petrological and experimental constraints on the evolution of Piton de la Fournaise magmas. Active Volcanoes of the Southwest Indian Ocean. Springer Publishing, 171–184
Roeder PL, Emslie RF (1970) Olivine-liquid equilibrium. Contrib Mineral Petrol 29:275–289. https://doi.org/10.1007/BF00371276
Rowe MC, Kent AJR, Nielsen RL (2007) Determination of sulfur speciation and oxidation state of olivine hosted melt inclusions. Chem Geol 236:303–322. https://doi.org/10.1016/j.chemgeo.2006.10.007
Saper LM, Stolper EM (2020) Controlled cooling-rate experiments on olivine-hosted melt inclusions: chemical diffusion and quantification of eruptive cooling rates on Hawaii and Mars. Geochem Geophys Geosystems 21:e2019GC008772. https://doi.org/10.1029/2019GC008772
Schiano P, David K, Vlastélic I, Gannoun A, Klein M, Nauret F, Bonnand P (2012) Osmium isotope systematics of historical lavas from Piton de la Fournaise (Réunion Island, Indian Ocean). Contrib Mineral Petrol 164:805–820. https://doi.org/10.1007/s00410-012-0774-0
Shorttle O, Moussallam Y, Hartley ME, Maclennan J, Edmonds M, Murton BJ (2015) Fe-XANES analyses of Reykjanes Ridge basalts: Implications for oceanic crust’s role in the solid Earth oxygen cycle. Earth Planet Sci Lett 427:272–285. https://doi.org/10.1016/j.epsl.2015.07.017
Sims KWW, Blichert-Toft J, Kyle PR, Pichat S, Gauthier P-J, Blusztajn J, Kelly P, Ball L, Layne G (2008) A Sr, Nd, Hf, and Pb isotope perspective on the genesis and long-term evolution of alkaline magmas from Erebus volcano, Antatctica. Volcanol Erebus Volcano Antarct 177:606–618. https://doi.org/10.1016/j.jvolgeores.2007.08.006
Sobolev AV, Nikogosian IK (1994) Petrology of long-lived mantle plume magmatism: Hawaii, Pacific, and Reunion Island, Indian Ocean. Petrology 2:111–144
Sobolev AV et al (2007) The amount of recycled crust in sources of mantle-derived melts. Science 316:412–417. https://doi.org/10.1126/science.1138113
Sorbadere F, Laurenz V, Frost DJ, Wenz M, Rosenthal A, McCammon C, Rivard C (2018) The behavior of ferric iron during partial melting of peridotite. Geochim Cosmochim Acta 239:235–254. https://doi.org/10.1016/j.gca.2018.07.019
Stolper DA, Keller CB (2018) A record of deep-ocean dissolved O2 from the oxidation state of iron in submarine basalts. Nature 553:323–327. https://doi.org/10.1038/nature25009
Stolper EM, Shorttle O, Antoshechkina PM, Asimow PD (2020) The effects of solid-solid phase equilibria on the oxygen fugacity of the upper mantle. Am Mineral. https://doi.org/10.2138/am-2020-7162
Stracke A (2012) Earth’s heterogeneous mantle: A product of convection-driven interaction between crust and mantle. Chem Geol 330–331:274–299. https://doi.org/10.1016/j.chemgeo.2012.08.007
Stracke A, Bizimis M, Salters VJM (2003) Recycling oceanic crust: Quantitative constraints. Geochem Geophys Geosystems. https://doi.org/10.1029/2001GC000223
Stracke A, Hofmann AW, Hart SR (2005) FOZO, HIMU, and the rest of the mantle zoo. Geochem Geophys Geosystems. https://doi.org/10.1029/2004GC000824
Vlastélic I, Lewin E, Staudacher T (2006) Th/U and other geochemical evidence for the Réunion plume sampling a less differentiated mantle domain. Earth Planet Sci Lett 248:379–393. https://doi.org/10.1016/j.epsl.2006.06.003
Widom E, Hoernle KA, Shirey SB, Schmincke HU (1999) Os Isotope systematics in the Canary Islands and Madeira: Lithospheric contamination and mantle plume signatures. J Petrol 40:279–296. https://doi.org/10.1093/pertoj/40.2.279
Zhang HL, Cottrell E, Solheid PA, Kelley KA, Hirschmann MM (2018) Determination of Fe3+/ΣFe of XANES basaltic glass standards by Mössbauer spectroscopy and its application to the oxidation state of iron in MORB. Chem Geol 479:166–175. https://doi.org/10.1016/j.chemgeo.2018.01.006
Zindler A, Hart S (1986) Chemical Geodynamics. Annu Rev Earth Planet Sci 14:493–571. https://doi.org/10.1146/annurev.ea.14.050186.002425
Acknowledgements
We are grateful to have received constructive reviews from Fabrice Gaillard and Brad Peters that improved this manuscript. We are also grateful for the careful handling of editor Dante Canil. We thank Pascal Richet for generously providing sample for this study. We thank C. Ma for assistance in microprobe analyses, and L. Saper for discussion of Fe and S redox, at the California Institute of Technology. We also thank A. Lanzirotti and M. Newville for assistance in beamline operations at Advanced Photon Source Argonne National Laboratory (APS ANL). Portions of this work were performed at GeoSoilEnviroCARS (The University of Chciago, Sector 13), Advanced Photon Source (APS), Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation – Earth Sciences (EAR-1634415) and Department of Energy – GeoSciences (DE-FG02-94ER14466). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dante Canil.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brounce, M., Stolper, E. & Eiler, J. The mantle source of basalts from Reunion Island is not more oxidized than the MORB source mantle. Contrib Mineral Petrol 177, 7 (2022). https://doi.org/10.1007/s00410-021-01870-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-021-01870-w