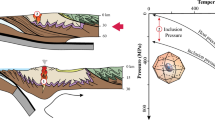
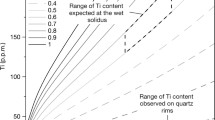
Abstract
Temperatures of crystallization for all or portions of three thin granitic pegmatite dikes in southern California are derived from feldspar solvus thermometry, with supporting data from the K/Cs ratio of K-feldspar, the extent of Al/Si order in K-feldspar, and the texture of granophyre found along the margins of dikes. Although K-feldspars become perthitic and increasingly ordered toward the centers of dikes, their ratio of K/Cs falls from margin to core along trajectories that reflect fractional crystallization from silicate melt without subsequent interaction with an aqueous solution in an open system. A few sporadic samples that record loss of Cs, and consequent rise in K/Cs, validate the test of fidelity that the perthites generally retain their igneous compositions. Feldspar solvus thermometry from these three dikes indicates that their pegmatite-forming melts crystallized at ~ 375–475 °C. Those low temperatures are consistent with the occurrence of granophyric plagioclase–quartz intergrowths along the borders of pegmatites, thick and thin, that arise from thermal quenching of their melts against much cooler host rocks, and hence at much shallower depths than the igneous sources of the pegmatite-forming melts. The temperature profiles are nearly isothermal across the pegmatites, but where variation exists, apparent temperatures are highest along their margins or in their central domains (cores). Plagioclase shows normal fractionation of decreasing An content from margins to center, which mimics the line of descent with cooling down the solidus and solvus surfaces. However, the fractionation trends in the feldspars are attributable to their isothermal crystallization far from the equilibrium of the liquidus at a highly undercooled state, not to crystallization upon cooling on the solidus surface.

(see London et al. 2012)


Boundaries between feldspar structural states and the names associated with them in quotation marks are from Kroll and Ribbe (1987)















Similar content being viewed by others
Notes
See Fig. 6 of London et al. (2012). The optical images therein are typical and representative of the new dikes studied in this work.
HEAT3D V. 4.15 program by Wohletz is available as freeware at https://kware-heat3d.software.informer.com/, last accessed March 2019. Inputs for this simulation are: (1) Rock: thermal conductivity = 3 W/m K, Clauser and Huegnes (1995); heat capacity = 1100 J/kg K, Robertson (1988), bulk density = 3000 kg/m3. (2) Magma: thermal conductivity = 2.5 W/m K, Zhao et al. (2016); heat capacity = 2200 J/kg K: Toplis et al. (2001).
Experiments are based on the total time at temperature, which includes the nucleation delay, the time from undercooling to the onset of crystallization, and the time after crystallization ceases due to the approach to equilibrium on the liquidus. The actual growth interval of crystals spans a fraction of that time.
References
Baker DR, Freda C (2001) Eutectic crystallization in the undercooled orthoclase–quartz–H2O system: experiments and simulations. Eur J Mineral 13:453–466
Blasi A, de Pol Blasi C (2000) Crystal structures of alkali feldspars from granitic pegmatites: a review. Memorie della Societa Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 30:73–109
Boyd B, Sirbescu ML, Matty DJ (2003) Crystallization temperatures of the Tin Mountain Pegmatite, Black Hills, South Dakota: insights from compositions of coexisting feldspars. In: Geological Society of America Programs with Abstracts vol 35, p 192 (abstr)
Brown JA (2001) Mineralogy and geochemistry of alkali feldspars from the Tanco pegmatite, southeastern Manitoba. M.Sc. thesis, University of Manitoba, Winnipeg, Manitoba
Brown JA, Martins T, Černý P (2017) The Tanco pegmatite at Bernic Lake, Manitoba. XVII. Mineralogy and geochemistry of alkali feldspars. Can Mineral 55:483–500
Burnham CW, Nekvasil H (1986) Equilibrium properties of granite pegmatite magmas. Am Mineral 71:239–263
Cameron EN, Jahns RH, McNair AH, Page LR (1949) Internal structure of granitic pegmatites. Econ Geol Mon 2:115
Carron JP, Lagache M (1980) Étude expérimentale du fractionnement des éléments Rb, Cs, Sr, et Ba entre feldspaths alcalins, solutions hydrothermales et liquides silicatés dans le système Q.Ab.Or.H2O à 2 kbar entere 700 et 800°C. Bull Minéral 103:571–578
Catlos EJ, Harrison TM, Kohn MJ, Grove M, Ryerson FJ (2001) Geochronologic and thermobarometric constraints on the evolution of the Main Central Thrust, central Nepal Himalaya. J Geophys Res 106B:16177–16204
Černý P (1974) Evolution of feldspars in granitic pegmatites. NATO Adv Study Inst Ser C Math Phys Sci 421:501–540
Černý P (2005) The Tanco rare-element pegmatite deposit, Manitoba: regional context, internal anatomy, and global comparisons. In: Linnen R, Sampson IM (eds) Rare-element geochemistry and mineral deposits. GAC short course notes, vol 17. Geological Association of Canada, pp 127–158
Černý P, Meintzer RE, Anderson AJ (1985) Extreme fractionation in rare-element granitic pegmatites: selected examples of data and mechanisms. Can Mineral 23:381–421
Chakoumakos BC, Lumpkin GR (1990) Pressure–temperature constraints on the crystallization of the Harding pegmatite, Taos County, New Mexico. Can Mineral 28:287–298
Clauser C, Huegnes E (1995) Thermal conductivity of rocks and minerals. Am Geophys Union Ref Shelf 3:105–126
Colombo F, Sfragulla J, del Gonzáles Tánago J (2012) The garnet–phosphate buffer in peraluminous granitic magmas: a case study from pegmatites of the Pocho District, Córdoba, Argentina. Can Mineral 50:1555–1571
Dingwell DB (1998) The glass transition in hydrous granitic melts. Phys Earth Planet Inter 107:1–8
Edgeworth R, Dalton BJ, Parnell T (1984) The pitch drop experiment. Eur J Phys 5:198–200
Elkins LT, Grove TL (1990) Ternary feldspar experiments and thermodynamic models. Am Mineral 75:544–559
Evensen JM (2001) The geochemical budget of beryllium in silicic melts and superliquidus, subliquidus, and starting state effects on the kinetics of crystallization in hydrous haplogranite melts. Unpublished Ph.D. dissertation, University of Oklahoma, Norman, Oklahoma, p 293
Evensen JM, London D (2002) Experimental silicate mineral/melt partition coefficients for beryllium, and the beryllium cycle from migmatite to pegmatite. Geochim Cosmochim Acta 66:2239–2265
Evensen JM, London D, Wendlandt RF (1999) Solubility and stability of beryl in granitic melts. Am Mineral 84:733–745
Felch M, Falster AU, Simmons WB (2016) Iron-bearing pollucite and tourmaline replacement of garnet in the garnet line in the Mt. Mica and Havey pegmatites, Western Maine. Can Mineral 54:1071–1086
Fenn PM (1986) On the origin of graphic granite. Am Mineral 71:325–330
Finch AA, Klein J (1999) The causes and petrological significance of cathodoluminescence emissions from alkali feldspars. Contrib Mineral Petrol 135:234–243
Fuhrman ML, Lindsley DH (1988) Ternary-feldspar modeling and thermometry. Am Mineral 73:201–215
Groat LA, Mulja T, Mauthner MHF, Ercit TS, Rausdepp M (2003) Geology and mineralogy of the Little Nahanni rare-element granitic pegmatites, Northwest Territories. Can Mineral 41:139–160
Hulsbosch N, Hertogen J, Dewaele S, André L, Muchez P (2014) Alkali metal and rare earth element evolution of rock-forming minerals from the Gatumba area pegmatites (Rwanda): quantitative assessment of crystal-melt fractionation in the regional zonation of pegmatite groups. Geochimica et Cosmochimica Acta 132:349–374
Jahns RH (1953) The genesis of pegmatites. I. Occurrence and origin of giant crystals. Am Mineral 38:563–598
Jahns RH (1982) Internal evolution of pegmatite bodies. In: Černý P (ed) Granitic pegmatites in science and industry. Mineral Association of Canada Short Course Handbook, vol 8, pp 293–327
Jahns RH, Burnham CW (1969) Experimental studies of pegmatite genesis: I. A model for the derivation and crystallization of granitic pegmatites. Econ Geol 64:843–864
Jambon A, Carron J-P (1976) Diffusion of Na, K, Rb and Cs in glasses of albite and orthoclase composition. Geochim Cosmochim Acta 40:897–903
Kargl A, Meyer M, Koza M, Schober H (2006) Formation of channels for fast-ion diffusion in alkali silicate melts: a quasielastic neutron scattering study. Am Soc Phys Phys Rev B 74:014304
Kroll H, Ribbe PH (1987) Determining (Al, Si) distribution and strain in alkali feldspars using lattice parameters and diffraction-peak positions: a review. Am Mineral 72:491–506
London D (1984) Experimental phase equilibria in the system LiAlSiO4-SiO2-H2O: a petrogenetic grid for lithium-rich pegmatites. Am Mineral 69:995–1004
London D (1990) Internal differentiation of rare-element pegmatites: a synthesis of recent research. In: Stein HJ, Hannah JL (eds) Ore-bearing granite systems; petrogenesis and mineralizing processes. Geological Society of America Special Paper 246, pp 35–50
London D (1999) Melt boundary layers and the growth of pegmatitic textures. Can Mineral 37:826–827 (abstr.)
London D (2008) Pegmatites. Can Mineral Spec Publ 10:368
London D (2009) The origin of primary textures in granitic pegmatites. Can Mineral 47:697–724
London D (2013) Crystal-filled cavities in granitic pegmatites: bursting the bubble. Rocks Miner 88:527–534
London D (2014a) A petrologic assessment of internal zonation in granitic pegmatites. Lithos 184:74–104
London D (2014b) Subsolidus isothermal fractional crystallization. Am Mineral 99:543–546
London D (2014c) Zonal structure of layered pegmatites: thin versus thick dikes. In: Geological Society of America Programs with Abstracts vol 46, p 751
London D (2015) Reply to Thomas and Davidson on “A petrologic assessment of internal zonation in granitic pegmatites” (London, 2014a). Lithos 212–215:469–484
London D (2016) Rare-element granitic pegmatites. In: Verplanck PL, Hitzman MW (eds) Rare earth and critical elements in major deposit types, vol 18. Society of Economic Geology Inc, Littleton, pp 165–193
London D (2018a) Ore-forming processes within granitic pegmatites. Ore Geol Rev 101:349–383
London D (2018b) Reading pegmatites: what quartz and feldspars say. Rocks Miner 93:320–336
London D (2019) Reading pegmatites: what pollucite says. Rocks Miner 94:420–425
London D, Morgan GB VI (2017) Experimental crystallization of the Macusani obsidian, with applications to lithium-rich granitic pegmatites. J Petrol 58:1005–1030
London D, Morgan GB VI, Hervig RL (1989) Vapor-undersaturated experiments in the system macusanite–H2O at 200 MPa, and the internal differentiation of granitic pegmatites. Contrib Mineral Petrol 102:1–17
London D, Morgan GBVI, Icenhower J (1998) Stability and solubility of pollucite in granitic systems at 200 MPa H2O. Can Mineral 36:497–510
London D, Morgan GBVI, Paul KA, Guttery BM (2012) Internal evolution of a miarolitic granitic pegmatite: the Little Three Mine, Ramona, California (USA). Can Mineral 50:1025–1054
Luth RW, Muncill GE (1989) Fluorine in aluminosilicate systems; phase relations in the system NaAlSi3O8–CaAl2Si2O8–F2O-1. Geochim Cosmochim Acta 53:1937–1942
Maner JL, London D, Icenhower JP (2019) Enrichment of manganese to spessartine saturation in granite-pegmatite systems. Am Mineral 104:1625–1637
Morgan GB VI, London D (1999) Crystallization of the Little Three layered pegmatite-aplite dike, Ramona District, California. Contrib Mineral Petrol 136:310–330
Morgan GB VI, London D (2005) Phosphorus distribution between potassic alkali feldspar and metaluminous haplogranite liquid at 200 MPa (H2O): the effect of undercooling on crystal-liquid systematics. Contrib Mineral Petrol 150:456–471
Morgan GB VI, London D (2012) Process of granophyre crystallization in the Long Mountain Granite, southern Oklahoma. Geol Soc Am Bull 124:1251–1261
Neves LJPF, Godinho MM (1999) Structural state of K-feldspar in some Hercynian granites from Iberia; a review of data and controlling factors. Can Mineral 37:691–700
Parsons I (1969) Subsolidus crystallization behaviour in the system KAlSi3O8–NaAlSi3O8–H2O. Mineral Mag 37:173–180
Parsons I, Magee CW, Allen CM, Shelley JMG, Lee MR (2009) Mutual replacement reactions in alkali feldspars II: trace element partitioning and geothermometry. Contrib Mineral Petrol 157:663–687
Rao BR, Rao AT (1990) Geothermometry of co-existing K-feldspar-plagioclase pairs from granites and pegmatites of Rampachoda, North Varam area in the eastern Ghats granulite belt. Indian J Geol 62:173–177
Robertson EC (1988) Thermal properties of rocks. In: US Geological Survey open file report 88–441, p 106
Rubin AM (1995) Getting granite dikes out of the source region. J Geophys Res 100B:5911–5929
Sanchez-Munoz L, Garcia-Guinea J, Zagorsky VY, Juwono T, Modreski PJ (2012) The evolution of twin patterns in perthitic K-feldspar from granitic pegmatites. Can Mineral 50:989–1024
Schulze F, Behrens H, Holtz F, Roux J, Johannes W (1995) Influence of water on the viscosity of a haplogranitic melt. Am Mineral 81:1155–1165
Seck HA (1971a) Koexistierende alkalifeldspite und plagioklase im system NaAlSi3O8–KAlSi3O8–CaAl2Si2O8–H2O bei temperaturen von 650°C bis 900°C. Neues Jahrbuch für Mineralogie Abhandlungen 115:315–345
Seck HA (1971b) Der einfluss des drucks auf die zusammensetzung koexistierender alkalifeldspiite und plagioklase. Contrib Mineral Petrol 31:67–86
Simmons WB, Falster AU, Felch M (2015) Pollucite from three distinct assemblages in the Mount Mica pegmatite, Paris, Oxford County, Maine, USA. In: 7th International symposium on granitic pegmatites, PEG 2015 Książ, Poland, p 96 (abstr)
Stilling A, Černý P, Vanstone PJ (2006) The Tanco pegmatite at Bernic Lake, Manitoba. XVI. Zonal and bulk compositions and their petrogenetic significance. Can Mineral 44:599–623
Swanson SE (1977) Relation of nucleation and crystal-growth to the development of granitic textures. Am Mineral 62:966–978
Thomas R, Davidson P (2015) Comment on “A petrologic assessment of internal zonation in granitic pegmatites” by David London (2014). Lithos 212:462–468
Toplis MJ, Gottmann J, Knoche R, Dingwell DB (2001) Heat capacities of haplogranitic glasses and liquids. Geochim Cosmochim Acta 65:1985–1994
Tuttle OF, Bowen NL (1958) Origin of granite in the light of experimental studies in the system NaAlSi3O8–KAlSi3O8–SiO2–H2O. Geol Soc Am Mem 74:153
Van Lichtervelde M, Holtz F, Melcher F (2018) The effect of disequilibrium crystallization on Nb–Ta fractionation in pegmatites: constraints from crystallization experiments of tantalite–tapiolite. Am Mineral 103:1401–1416
Watson EB (1975) Diffusion of cesium ions in H2O-saturated granitic melt. Science 205:1259–1260
Webber KL, Simmons WB, Falster AU, Foord EE (1999) Cooling rates and crystallization dynamics of shallow level pegmatite–aplite dikes, San Diego County, California. Am Mineral 84:708–717
Wen S, Nekvasil H (1994) SOLVCAL: an interactive program package for calculating ternary feldspar solvus and two-feldspar geothermometry. Comput Geosci 20:1025–1040
Zhao XG, Wanga J, Chena F, Li PF, Ma LK, Xie JL, Liua YM (2016) Experimental investigations on the thermal conductivity characteristics of Beishan granitic rocks for China’s HLW disposal. Tectonophysics 683:124–137
Acknowledgements
We thank George B. Morgan VI for microprobe analyses of the Palomar feldspars. Blue Sheppard, Wallace Kleck, and Louis B. Spaulding Jr. (dec.) made this project possible by providing the dike sections studied. The electron microprobe laboratory is funded by the Office of the Vice President of Research at the University of Oklahoma, with most recent Grants for upgrades from the National Science Foundation (EAR-1401940) and the U.S. Department of Energy (Laboratory Equipment Donation Program, item 8975793213S7130). Research support came from National Science Foundation Grants EAR-0946322, EAR-1623110, and from the Norman R. Gelphman professorship to D. L. We thank an anonymous reviewer comments and meticulous editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Timothy L. Grove.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
London, D., Hunt, L.E., Schwing, C.R. et al. Feldspar thermometry in pegmatites: truth and consequences. Contrib Mineral Petrol 175, 8 (2020). https://doi.org/10.1007/s00410-019-1617-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-019-1617-z