Abstract
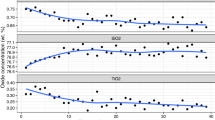
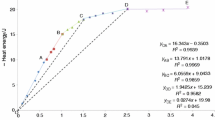
The activity coefficient of aqueous silica was determined at 800 °C and 12 kbar by measuring the concentration of total dissolved aqueous silica (SiO2(t)) in H2O in equilibrium with four buffering mineral assemblages: quartz (Q), kyanite–corundum (KC), enstatite–forsterite (EF), and forsterite–rutile–geikielite (FRG). SiO2(t) concentrations were determined by reversed equilibration of synthetic minerals with H2O using a weight gain/weight loss technique, yielding molalities of SiO2(t) (m s) of Q, 1.413(1); KC, 0.984(43); EF, 0.276(9); FRG, 0.079(1) (1σ errors). The solubility data can be fitted well according to the homogeneous equilibrium 2 SiO2 monomers (m) = 1 Si2O4 dimer (d) assuming ideal mixing, such that the equilibrium constant (K md ) is K md =X d /X m 2, where X represents the mole fraction of SiO2(t) occurring as the subscripted species. This formulation is valid irrespective of the hydration states of the silica species because of the small silica concentration at these conditions (≤2.5 mol%). For a standard state of unit activity of the hypothetical pure monomer solution at the P and T of interest, the monomer–dimer model leads to γ s =X m /X s and
where γ s and X s are the activity coefficient and mole fraction of total silica. Our data yield \( {K_{{md}} = 155^{{ + 25}}_{{ - 39}} } \) at 800 °C and 12 kbar, in excellent agreement with results from in situ Raman spectroscopy of a quartz-saturated solution at the same P–T. In the system SiO2–H2O, a solution in equilibrium with quartz at 800 °C, 12 kbar, contains 2.5 mol% silica, of which 70% occurs in dimers, and γ s is small (0.30). Even at the low concentration of the FRG buffer (0.1 mol%), the activity coefficient is only 0.75 and the dissolved silica is substantially polymerized (25%).




Similar content being viewed by others
References
Bebout GE, Barton MD (1989) Fluid flow and metasomatism in a subduction zone hydrothermal system: Catalina Schist terrane, California. Geology 17:976–980
Berman RG (1988) Internally consistent thermodynamic data for minerals in the system Na2O–K2O–CaO–MgO–FeO–Fe2O3–Al2O3–SiO2–TiO2–H2O–CO2. J Petrol 29:445–522
Ferry JM (1994) A historical review of metamorphic fluid flow. J Geophys Res 99:15487–15498
Ferry JM, Newton RC, Manning CE (2002) Experimental determination of the equilibria: rutile + magnesite = geikielite + CO2 and zircon + 2 magnesite = baddeleyite + 2CO2. Am Mineral 87:1342–1350
Fournier RO, Potter RW II (1982) An equation correlating the solubility of quartz in water from 25° to 900 °C at pressures up to 10,000 bars. Geochim Cosmochim Acta 46:1969–1973
Harlov DE, Newton RC (1992) Experimental determination of the reaction 2magnetite +2kyanite + 4quartz = 2almandine + O2 at high pressure on the magnetite-hematite buffer. Am Mineral 77:558–564
Haselton HT, Sharp WR, Newton RC (1978) CO2 fugacity at high temperatures and pressures from experimental decarbonation reactions. Geophys Res Lett 5:753–756
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrologic interest. J Metamorph Geol 16:309–343
Kennedy GC, Wasserburg GJ, Heard HC, Newton RC (1962) The upper three-phase region in the system SiO2–H2O. Am J Sci 260:501–521
Manning CE (1994) The solubility of quartz in the lower crust and upper mantle. Geochim Cosmochim Acta 58:4831–4839
Manning CE (1995) Phase-equilibrium controls on SiO2 metasomatism by aqueous fluids in subduction zones: reaction at constant pressure and temperature. Int Geol Rev 37:1074–1093
Manning CE (1998) Fluid composition at the blueschist–eclogite transition in the model system Na2O–MgO–Al2O3–SiO2–H2O–HCl. Swiss Bull Mineral Petrol 78:225–242
Masson CR (1965) An approach to the problem of ionic distribution in liquid silicates. Proc R Soc Lond A287:201–221
Newton R C, Manning CE (2000a) Metasomatic phase relations in the system CaO–MgO–SiO2–H2O–NaCl at high temperatures and pressures. Int Geol Rev 42:152–162
Newton RC, Manning CE (2000b) Quartz solubility in concentrated aqueous NaCl solutions at deep crust–upper mantle metamorphic conditions: 2–15 kbar and 500–900 °C. Geochim Cosmochim Acta 64:2993–3005
Newton R C, Manning CE (2002) Solubility of enstatite + forsterite in H2O at deep crust/upper mantle conditions: 4 to 15 kbar and 700 to 900 °C. Geochim Cosmochim Acta 66:4165–4176
Shen AH, Keppler H (1997) Direct observation of complete miscibility in the albite–H2O system. Nature 385:710–712
Stolz AJ, Davies GR (1989) Metasomatized lower crustal and upper mantle xenoliths from north Queensland: chemical and isotopic evidence bearing on the composition and source of the fluid phase. Geochim Cosmochim Acta 53:649–660
Walther JV, Helgeson HC (1977) Calculation of the thermodynamic properties of aqueous silica and the solubility of quartz and its polymorphs at high pressures and temperatures. Am J Sci 277:1315–1351
Walther J, Orville PM (1983) The extraction-quench technique for determination of the thermodynamic properties of solute complexes: application to quartz solubility in fluid mixtures. Am Mineral 68:731–741
Zhang Y-G, Frantz JD (2000) Enstatite–forsterite–water equilibria at elevated temperatures and pressures. Am Mineral 85:918–925
Zotov N, Keppler H (2000) In-situ Raman spectra of dissolved silica species in aqueous fluids to 900 °C and 14 kbar. Am Mineral 85:600–603
Zotov N, Keppler H (2002) Silica speciation in aqueous fluids at high pressures and high temperatures. Chem Geol 184:71–82
Acknowledgements
This research was funded by NSF EAR-990583. We thank John Walther and Andy Shen for helpful reviews of the manuscript, and George Jarzebinski for assistance with SEM petrography.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: T.L. Grove
Rights and permissions
About this article
Cite this article
Newton, R.C., Manning, C.E. Activity coefficient and polymerization of aqueous silica at 800 °C, 12 kbar, from solubility measurements on SiO2-buffering mineral assemblages. Contrib Mineral Petrol 146, 135–143 (2003). https://doi.org/10.1007/s00410-003-0483-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-003-0483-9