Abstract
Airway nerves regulate vital airway functions including bronchoconstriction, cough, and control of respiration. Dysregulation of airway nerves underlies the development and manifestations of airway diseases such as chronic cough, where sensitization of neural pathways leads to excessive cough triggering. Nerves are heterogeneous in both expression and function. Recent advances in confocal imaging and in targeted genetic manipulation of airway nerves have expanded our ability to visualize neural organization, study neuro-immune interactions, and selectively modulate nerve activation. As a result, we have an unprecedented ability to quantitatively assess neural remodeling and its role in the development of airway disease. This review highlights our existing understanding of neural heterogeneity and how advances in methodology have illuminated airway nerve morphology and function in health and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Airway nerves serve critical functions in the upper and lower respiratory tract including regulation of breathing and control of bronchoconstriction and cough. Both afferent and efferent fibers contribute to these functions and represent numerous neuronal subsets, each contributing discrete input to the regulation of airway functions. Numerous methods for classifying airway nerve subtypes have been proposed based on neuronal structure, expression, or function, yet no single classification scheme fully encapsulates airway neuronal diversity. Single-cell RNA sequencing techniques have highlighted this diversity by identifying at least 18 unique transcriptomic subtypes of sensory nerves alone [1]. Nerve subtypes frequently exhibit overlapping expression of receptors, neurotransmitters, and neuropeptides, further underscoring the challenge of creating a unifying classification scheme [2]. However, recent technological innovations in confocal microscopy and advances in genetic manipulation have provided new opportunities for studying nerve structure, expression, and function for both common and rare neuronal subtypes in the lungs. Here, we describe recent insights derived from studies using novel methods, with a focus on airway neural organization in healthy lungs and the role of neural remodeling in the pathogenesis of chronic cough.
Neurologic Origins of Chronic Cough
Cough is a protective response that clears pathogens and mucus from airways and is regulated by airway sensory nerves [3]. To produce an effective cough, sensory input must be integrated in the brainstem to evoke responses in skeletal nerves and efferent airway nerves produce a deep inspiration followed by forced exhalation against a closed glottis [4]. The necessity of effective coughing to lung health is underscored by the increased frequency of pneumonia in conditions where cough is impaired [5].
Unlike protective cough, chronic cough represents a pathologic state that no longer serves a physiologic role. Chronic cough is a central feature that develops in a myriad of lung diseases [3]. That chronic cough is shared by diseases with disparate pathologies, such as asthma (an inflammatory airway disease) and idiopathic pulmonary fibrosis (an alveolar fibrosing disease), highlights the significant role that dysregulated airway nerves play in the clinical manifestations of lung disease. Patients with chronic cough frequently report an urge to cough coupled with an irritation or “itch” sensation in the throat and a heightened sensitivity to environmental triggers such as cold air or perfumes. These symptoms, which have been termed “cough hypersensitivity,” develop due to sensitization of neuronal pathways that govern cough and contribute to excessive cough triggering [6].
Cough challenge studies suggest that neuronal sensitization is a heterogeneous process that results in distinct neurophenotypes, as reflected by differing cough responses to inhaled stimuli between airway diseases [7]. For example, cough sensitivity to inhaled capsaicin (an agonist of neuronal transient receptor potential (TRP) V1 was similar between patients with chronic obstructive pulmonary disease and chronic idiopathic cough, while sensitivity to inhaled prostaglandin E2 was significantly different. These unique cough neurophenotypes are predicted to result from different mediators and mechanisms driving development of each disease.
Organization of Airway Innervation in Healthy Lungs
Sensory Afferent Innervation of the Larynx and Lower Airway
Sensory innervation of the lower airways, extending from the larynx proximally to the distal terminal bronchioles, is provided primarily by fibers contained with the vagus nerves, with minor contributions provided by sensory neurons from the thoracic dorsal root ganglia [2, 8, 9]. Vagal sensory nerve cell bodies are contained within the jugular (superior) and the nodose (inferior) ganglia, collectively termed the vagal ganglia, located at the base of the skull [10]. These ganglia have distinct embryological origins and targets. Jugular nerves are derived from neural crest cells and primarily innervate the trachea and large airways, whereas nodose nerves are derived from epibranchial placodes and provide innervation to distal airways and lungs [11]. Sensory axons terminate within all major compartments of the airways including the epithelium, subepithelium, and smooth muscle, while also providing discrete innervation to airway mucus glands, autonomic ganglia, alveolar capillary beds, and other airway structures [12,13,14].
Sensory nerves can be broadly classified as mechanoreceptors or chemoreceptors based on their responsiveness to mechanical or chemical stimuli. Mechanoreceptors are typically larger myelinated fibers that are highly sensitive to touch, whereas chemoreceptors (also termed nociceptors or C fibers) are typically small-diameter, unmyelinated fibers that express a wide array of receptors and ion channels capable of detecting inhaled and endogenous noxious compounds, and changes in pH, temperature, and osmolarity [10, 15,16,17,18]. Receptors with specific relevance to cough (both to cough triggering and in the pathogenesis and potential treatment of chronic cough) include P2X3 purinergic receptors, voltage-gated sodium channels (NaV), bradykinin receptors, and TRP channels (discussed below) [19, 20]. Mechanoreceptors can be further subclassified as slowly adapting and rapidly adapting based on their speed of adaptation to sustained stimuli and their ability to modulate respiratory patterns and cough responses. Sensory nerve input is transmitted to the paratrigeminal nucleus (jugular) and nucleus of the solitary tract (nodose) within the brainstem [21,22,23]. Input from both mechanoreceptors and nociceptors can trigger cough, during which the respiratory pattern generator of the brainstem switches from a rhythmic breathing pattern to a cough pattern. Sensory input is also transmitted to efferent airway nerves to induce reflex bronchoconstriction and to higher-order cortical neurons where conscious perception of cough and cough suppression centers may modulate coughing.
Efferent Innervation of the Lower Airways
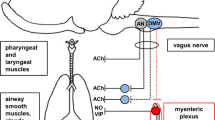
The primary efferent innervation of the airways is provided by cholinergic parasympathetic nerves, which provide the dominant control of bronchoconstriction [24]. Preganglionic parasympathetic neurons originate in the dorsal motor nucleus and nucleus ambiguus in the brainstem, travel within the vagus nerves (alongside sensory afferents), and synapse on postganglionic nerves contained in airway ganglia seated in the walls of the trachea and extrapulmonary bronchi [25,26,27,28]. Post-ganglionic processes branch extensively throughout the tracheobronchial tree to terminate on submucosal glands [12, 29], blood vessels [13], and most prominently, airway smooth muscle [30], where they release acetylcholine to induce smooth muscle contraction via M3 muscarinic receptor activation. Acetylcholine also binds prejunctional M2 muscarinic receptors, which provides an inhibitory feedback mechanism limiting further acetylcholine release [31,32,33,34,35,36]. Parasympathetic acetylcholine release is triggered by input from the cortex and by direct stimulation from sensory nerves in the brainstem [14, 37]. Bronchoconstriction resulting from sensory nerve-mediated parasympathetic nerve activation is termed “reflex bronchoconstriction.” Reflex bronchoconstriction has been demonstrated in both humans and animals, and in response to a variety of sensory nerve stimuli including histamine [37], methacholine [38], allergen [39], cold air [40], and exercise [41].
In addition to parasympathetic nerves, sympathetic and non-adrenergic non-cholinergic nerves provide additional efferent innervation of the lower airways [42]. In humans, sympathetic fibers principally innervate airway vasculature, with essentially no direct input to airway smooth muscle (in contrast to the sympathetic innervation of smooth muscle in mice) [43]. In contrast, non-adrenergic non-cholinergic (NANC) nerves induce airway smooth muscle relaxation through release of nitric oxide (NO) and vaso-intestinal peptide (VIP) [44,45,46,47].
Advanced Methods for Studying Airway Innervation
Confocal Microscopy Illuminates 3-Dimensional Complexity of Sensory Innervation
Airway sensory nerves form complex, 3-dimensional structures that can span hundreds of histologic tissue sections. This complexity has made studying the morphology of airway nerves in individual tissue sections challenging. Heterogenous expression of receptors and neuropeptides by airway sensory nerves has further complicated quantitative assessments of nerve morphology [48]. However, advances in confocal imaging and immunohistochemistry have bridged this technological gap by capturing high-resolution, 3-dimensional image Z-stacks of airway structures using whole-mount tissues that do not require tissue sectioning. When paired with tissue optical clearing, where airway specimens or whole lungs are rendered transparent by an optical clearing reagent, image Z-stacks can extend through entire organs, limited only by the optical constraints of the confocal objectives (Fig. 1a, b) [49, 50].
Tissue optical clearing and high-resolution confocal microscopy enable quantitative modeling of airway nerve morphology. a Mouse trachea immunostained and cleared using an optical clearing reagent to render tissue transparent. b Immunolabeling of transparent airway tissues followed by confocal imaging provides detailed visualization of airway nerves. Orange: pan-neuronal marker AbPGP9.5; Green: channelrhodopsin-CH2; Blue: nuclear stain DAPI. Scale bar: 500 µm. c Epithelial sensory nerves in a human bronchiole immunostained for PGP9.5 (green), NFHC (magenta), and neuropeptide substance P (red). Scale bar: 10 µm d 3D nerve model based on PGP9.5-positive voxels in C using Imaris software; Scale bar: 20 µm. e Parasympathetic ganglion in optogenetic mouse trachea immunostained for PGP9.5 (yellow), channelrhodopsin-CH2 (turquoise) and neuropeptide substance P (magenta). Scale bar: 20 µm. f Parasympathetic ganglion in human lung immunostained for PGP9.5 (green), substance P (red), and TRPV1 (magenta). Scale bar: 30 µm. PGP9.5 protein gene product 9.5, CH2 channelrhodopsin-2 expressed on parasympathetic ganglia, TRPV1 transient receptor potential vanilloid subtype 1, NFHC neurofilament heavy chain
Confocal techniques have illuminated a remarkable degree of three-dimensional neural complexity in both human airways [51,52,53] and animal models, including rat [54], pig [55], rabbit [56], guinea pig [57], and mouse [49, 50]. Nerves are interposed within and around virtually all airway structures and in all tissue layers (e.g., epithelium, subepithelium, smooth muscle, etc.) [58]. Epithelial sensory nerves, for example, extend from subepithelial roots to form a lattice of branching nerve terminals among airway epithelial cells in close juxtaposition to airways, where they detect impacting particles and noxious compounds (Fig. 1c, d). Sensory nerve density (i.e., total nerve length) and complexity (i.e., nerve branching) vary by airway location and are tissue-compartment specific, with both density and complexity decreasing from proximal trachea to bronchi, and from the dorsal to ventral aspect of the airway [49, 50]. Epithelial nerve complexity is also greatest at airway branch points, where inhaled particles are most likely to impact, and surrounding airway parasympathetic ganglia embedded within the airway wall (Fig. 1e, f) and among specialized cells embedded within the epithelial layer termed neuroepithelial bodies (NEBs) [59]. Collectively, NEBs and the nerve axons surrounding them are termed pulmonary neuroendocrine cells (PNECs), and are composed of multiple TRPV1 and substance P-expressing nociceptive C fibers, and cholinergic neurons [60], suggesting that these cells functionally serve mechanosensory, chemosensory, and cholinergic roles. PNECs are sparsely and randomly distributed throughout human airways [61].
Fluorescence Labeling Highlights Axonal Organization
As nerve axons travel deeper into tissues (i.e., from epithelium to subepithelium and smooth muscle), they frequently join larger nerve bundles consisting of a mix of sensory and efferent fibers. Techniques have been developed to trace nerve axons to their termination, including the application of lipophilic dyes (e.g., carbocyanine dye DiI) [62], horseradish peroxidase [63], and wheat germ agglutin [64]; methods that involve uptake and transport of tracers along axons to enable visualization of nerve course and synaptic organization. Su et al. recently combined retrograde tracing methods, immunohistochemistry, and confocal imaging to show that central projections of TRPV1 and substance P-positive neurons within vagal ganglia have both shared and distinct synaptic targets in airways, including within airway smooth muscles, along lymphatic, and surrounding alveoli [65]. Confocal immunostaining has also demonstrated patterns of overlapping expression patterns on sensory neurons. For example, on nociceptors, the most commonly expressed receptors and peptides included Trpv1 (78%), Piezo1 (74%), Piezo2 (69%), and substance P (57%), followed by Calb1 (45%), Trpa1 (48%), and VIP (24%) . Receptors and peptides frequently colocalize (i.e., dual TRPV1 + and substance P + sensory neurons), with each unique combination of co-expression representing a small portion of total nerves overall [57, 66].
Neuronal organization and expression have been defined using Cre-lox-based genetic reporter mice coupled with fluorescent proteins. Using Pirt+, 5HT3+, substance P+, and TRPV1 + reporters, this method demonstrated relative contributions of each neuronal subtype to the innervation of airway targets [67]. Piezo2 reporters have also been used to elucidate their functional roles in detecting pulmonary stretch [68].
Multi-color nerve labeling is an alternative method for axonal tracing. Unlike retrograde tracers or Cre reporter mice, which label nerves originating from a common site (i.e., airway lumen) or expressing a specific promoter with a single color, multi-color nerve labeling provides a distinct fluorescent color for each nerve process, enabling distinction of individual axons in close proximity and tracing of individual nerves to their target of innervation [69]. Multi-color labeling has been used to study neurons in the brain (e.g., Brainbow mice [70]), where fluorophore expression was driven by the Thy1 promoter. Peripheral nerves had not been studieds in this manner due to the absence of Thy1 promoter expression by peripheral neurons. We used a modified technique involving simultaneous injection of three neurotrophic adeno-associated virus (AAV) vectors tagged with a distinct fluorophore to produce a spectrum of colors in airway neurons [69]. Random viral transduction within each neuron produces different ratios of fluorophore expression to enable distinction and tracing of individual nerve axons. When paired with conventional immunohistochemistry, the morphology of specific nerves, such substance P, neuronal NOS-, and TH-expressing neurons, can be traced to their termination.
Confocal Studies of Axonal Development During Embryogenesis
During embryogenesis, airway neuronal outgrowth is closely associated with airway elongation and airway smooth muscle proliferation [71]. These primitive airway tubules are coated by a dense neural plexus overlying smooth muscle, which by the canicular phase, forms two distinct bronchial trunks giving rise to varicosed fibers and discrete airway ganglia [72]. Similar patterns of neuronal elongation and branching have been demonstrated in the pig, rabbit, and mouse fetal lung, where neural tissue is a dominant feature of the developing lung [61, 72,73,74].
Vagal sensory input supplies an abundance of fibers to cholinergic airway ganglia precursors as well. These ganglia, which originate from neuroblasts along the wall of the epithelial tubules during the pseudoglandular stage, coalesce and become increasingly enveloped by glial fibrillary acid-positive sheaths. Ganglionic neurons transition to a cholinergic phenotype from the canalicular stage onward, further increasing in size during the saccular phase and during early post-natal development [73].
Transgenic Models for Testing Airway Nerve Function
Testing the function of activated airway nerves has historically required electrical stimulation, either via electrodes attached to nerve bundles in vivo (e.g., the vagus nerve trunks) or by applying electrical currents across isolated airway segments ex vivo [75], or through the application of pharmacologic agonists. While these techniques contributed significantly to our understanding of neural control of airway function, their readouts were limited by a lack of selectivity for neuronal subtypes. Recent applications of transgenic and Cre-recombinase-based methods, such as optogenetics and in vivo calcium fluorescence, have significantly advanced our ability to manipulate and measure the function of neuronal subtypes.
Optogenetics involves genetic insertion of photosensitive ion channels into specific neuronal subpopulations, enabling targeted nerve activation or inhibition using light [76]. While discovery of opsin-based channels is now over 20 years ago, genetic insertion techniques and channel options continue to expand, providing increasingly selective control of nerve function. Our lab has applied this approach to provoke or inhibit nerve-mediated bronchoconstriction in vivo by inserting nerve-activating channelrhodopsin and nerve-inhibiting halorhodopsin channels into efferent choline-acetyltransferase-expressing cholinergic parasympathetic nerves and into advillin- and tac1-expressing sensory nerves [77, 78]. Similarly, optogenetic activation of TRPV1- and S1PR3-positive sensory nerves stimulated bronchoconstriction in allergen-sensitized mice [79]. Activation of P2RY1-expressing sensory neurons triggered a series of reflexes designed to prevent aspiration, including pharyngeal swallowing, apnea, and vocal fold adduction [80, 81], while Piezo2-expressing sensory neurons, which often also co-express P2RY1, produced sustained apnea upon optogenetic light stimulation without pharyngeal and vocal cord reflexes, suggesting that Piezo2 neurons provide mechanosensory feedback of lung stretch during physiologic respiration [68].
Cre-lox recombination has also been used to insert calcium-sensitive fluorophores into neurons to study nerve activation in vivo at a single-cell resolutionwith two-photon microscopy [82]. In this study, Pirt-cre mice in which cre recombinase is expressed in all vagal neurons were crossed with R26‐GCaMP6s to create a strain that expresses a calcium-sensitive fluorophore in vagal sensory neurons. The effects of the lipid agonist sphingosine‐1‐phosphate (S1P), which is elevated in asthma, were then tested in vivo. Approximately 80% of vagal sensory neurons responded to SP1 via S1PR3 receptors, suggesting that elevated S1P levels in inflammatory conditions like asthma contribute to increased neuronal activation in diseased lungs.
Neuronal Remodeling and Neuro-immune Interactions—Implications for Chronic Cough Pathogenesis
Neural Sensitization Contributes to Excessive Cough
Several mechanisms have been identified, which may contribute to neuronal sensitization in chronic cough, including increased nociceptor sensitivity, de novo expression of nociceptors and neuropeptides by sensory neurons, increased airway epithelial nerve density, and increased release of endogenous cough-triggering molecules in airways [83]. While most of these mechanisms are derived from animal models, we recently demonstrated in bronchoscopic human airway samples using tissue optical clearing and confocal microscopy that airway epithelial sensory nerve density is doubled in patients with chronic cough compared to healthy airways [53]. In some samples, sensory neuropeptide substance P was also increased although not uniformly in the chronic cough cohort, in line with cough challenge studies suggesting that heterogeneous neuronal remodeling events may underlie the development of clinical symptoms [7].
Substance P augments cough responses by lowering neuronal activation thresholds [84, 85]. However, its role in chronic cough has been in doubt since an initial clinical trial of a substance P receptor (neurokinin 1 and 2) antagonist failed to reduce cough frequency [86]. Since that study, a second family of substance P receptors (mas-related g-protein coupled receptors, Mrgprs) has been discovered, which has been linked to the generation of itch; a sensory nerve-mediated process in skin with many similarities to cough [87]. This pathway, coupled with the identification of distinct cough neurophenotypes and our finding that substance P is increased in human airways, suggest that neurokinins require a fresh examination as a therapeutic target. Indeed, two recent studies of the NK-1 receptor antagonist, aprepitant, reported a decrease in cough frequency compared to placebo in patients with lung cancer and chronic cough [88, 89]. A second NK-1 receptor antagonist is also under clinical investigation [90]. Whether this approach will be broadly applicable across diseases or more targeted for specific populations awaits further study.
Several sensory receptors have also been implicated in chronic cough generation. Foremost are P2X3 purinoreceptors, which are expressed by approximately 1/3 of nodose sensory neurons [91, 92] and are activated by ATP, an endogenous mediator released during times of cell stress. In diseased lungs, cough responses to inhaled ATP are increased [93,94,95]. Moreover, P2X3 antagonists in phase 2 and 3 trials have demonstrated reductions in cough frequency [96,97,98,99,100,101,102]. If approved for clinical use, P2X3 receptor antagonists would represent the first-targeted therapy approved for chronic cough.
How P2X3 signaling is modulated in disease is an area of active interest. Increased extracellular ATP has been reported in asthma and chronic obstructive pulmonary disease, which may contribute to P2X3-mediated cough [103, 104]. However, this finding would not explain the increased sensitivity to inhaled ATP in chronic cough [95]. Rather, we hypothesized that neuronal P2X3 expression is increased by airway inflammation. To test this postulate, we quantified airway neuronal P2X3 expression in a mouse model of eosinophilic asthma (a disease frequently associated with chronic cough). In these mice, neuronal P2X3 expression was significantly increased compared control animals, suggesting modulation of P2X3 expression may underlie development of ATP sensitivity in some cases [105]. Modulation of endogenous ATP release may also occur, either through alterations in the number or function of ATP-releasing Pannexin-1 channels on structural and inflammatory cells directly, or indirectly via modulation of pathways that regulate Pannexin-1 function, as suggested by Bonvini et al. who reported that TRPV4 agonist stimulated Pannexin-1 ATP release to evoke cough [106].
TRP channels, including TRPV4 as well as TRPV1, TRPA1, and others, are a family of transmembrane proteins that detect a wide array of cough-provoking irritants [107,108,109,110,111]. Cough studies in guinea pigs have revealed a positive correlation between cough frequency and TRPA1 and TRPV1 expression [112], and increased TRPV1 channel expression has been demonstrated in airways of humans with chronic cough [113, 114]. In patients with asthma and in those with chronic obstructive pulmonary disease (both conditions associated with chronic cough), TRPV1 expression and cough responses to TRPV1 agonists are increased [7]. In animal models, mechanoreceptors express TRPV1 de novo after allergen exposure and virus infection [115,116,117], possibly due to induction of the neurotrophin brain-derived neurotrophic factor (BDNF) [115].
Despite a clear role for TRP channels in triggering cough and TRP channel antagonists’ efficacy in blocking evoked cough responses, multiple TRP antagonists have failed to reduce cough frequency in chronic cough clinical trials [118,119,120]. This apparent discrepancy highlights the challenge in developing anti-tussives that block pathologic cough while preserving protective cough.
Neuro-immune Interactions Result in Dysregulated Airway Function
Chronic cough is a common feature in over 100 distinct diseases, many of which are characterized by the influx of inflammatory cells into airways. As an example, asthma is an inflammatory airway disease characterized by excessive bronchoconstriction and in many cases, chronic cough, with increased sensitivity to inhaled irritants [121]. In asthma, airway eosinophils, which are abundant in a majority of patients, migrate to nerves due to neuronal release of the eosinophil chemoattractant eotaxin [122,123,124]. Eosinophil’s interactions with nerves have profound effects on both afferent and efferent pathways. Airway eosinophils were associated with increased epithelial sensory nerve density in bronchoscopic airway samples from humans with asthma and were demonstrated to mediate sensory hyperinnervation in mice (quantified using confocal microscopy) [52]. In mice, increased sensory nerve density develops after chronic allergen-induced eosinophilia (i.e., house dust mite allergen exposure for 8 weeks) and in offspring exposed to maternal asthma in utero, suggesting hyperinnervation develops due to prenatal programming, predisposing an individual to lung disease later in life [125, 126]. These morphologic changes in airway nerves, which are akin to those seen in idiopathic chronic cough patients [53], increase bronchoconstriction evoked by sensory nerve activation and were associated with increased sensitivity to environmental irritants [52]. Eosinophils also exacerbate efferent parasympathetic nerve control of bronchoconstriction [122, 127, 128]. Thus, both bronchoconstriction and cough, which are cardinal signs of asthma, result from inflammatory cell effects on each limb of airway innervation.
Eosinophil proximity to nerves is critical to the development of nerve dysfunction. To study the effects of eosinophil proximity on nerve structure and function, we paired in vivo measurements of bronchoconstriction using optogenetic mice with confocal imaging to quantify spatial interactions between leukocytes and their effects on neuronal subtypes. We demonstrated that the density of tissue eosinophils is significantly increased around airway nerves, which correlates with increased neuronally-mediated bronchoconstriction [77, 78]. The combined effects of eosinophil interactions with nerves, coupled with pre-existing airway hyperinnervation, were profound, resulting in fatal bronchoconstriction in a mouse model of asthma [125]. Thus, structural remodeling coupled with physical interactions with eosinophils severely dysregulates neural control of airway tone.
Conclusions
Airway nerves are heterogeneous, with overlapping patterns for receptors and protein expression that define their functional role in regulating cough, bronchoconstriction, respiration, and other functions. Neuronal remodeling underlies the development of airway disorders, including most prominently, chronic cough. Advances in confocal imaging and genetic methods have expanded our understanding of the function and morphology of neuronal subtypes, while enabling quantitative analyses of neuronal remodeling and neuro-immune interactions. These results offer new insights into mechanisms of disease pathogenesis and potential treatment targets, for which targeted therapies in chronic cough are urgently needed.
References
Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P (2019) An atlas of vagal sensory neurons and their molecular specialization. Cell Rep 27:2508-2523.e2504
Mazzone SB, Undem BJ (2016) Vagal afferent innervation of the airways in health and disease. Physiol Rev 96:975–1024
Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo RC, Hilton BM, Kantar A, Lai K, McGarvey L, Rigau D, Satia I, Smith J, Song WJ, Tonia T, van den Berg JWK, van Manen MJG, Zacharasiewicz A (2020) ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 55:58
Canning BJ (2009) Central regulation of the cough reflex: therapeutic implications. Pulm Pharmacol Ther 22:75–81
Niimi A, Matsumoto H, Ueda T, Takemura M, Suzuki K, Tanaka E, Chin K, Mishima M, Amitani R (2003) Impaired cough reflex in patients with recurrent pneumonia. Thorax 58:152–153
Chung KF, McGarvey L, Song WJ, Chang AB, Lai K, Canning BJ, Birring SS, Smith JA, Mazzone SB (2022) Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 8:45
Belvisi MG, Birrell MA, Khalid S, Wortley MA, Dockry R, Coote J, Holt K, Dubuis E, Kelsall A, Maher SA, Bonvini S, Woodcock A, Smith JA (2016) Neurophenotypes in airway diseases. insights from translational cough studies. Am J Respir Crit Care Med 193:1364–1372
Coleridge JC, Coleridge HM (1984) Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99:1–110
Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M (2004) Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556:905–917
Ricco MM, Kummer W, Biglari B, Myers AC, Undem BJ (1996) Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496(Pt 2):521–530
Baker CV, Schlosser G (2005) The evolutionary origin of neural crest and placodes. J Exp Zool B 304:269–273
Wine JJ (2007) Parasympathetic control of airway submucosal glands: central reflexes and the airway intrinsic nervous system. Auton Neurosci 133:35–54
Coleridge HM, Coleridge JC (1994) Neural regulation of bronchial blood flow. Respir Physiol 98:1–13
Canning BJ (1985) Reflex regulation of airway smooth muscle tone. J Appl Physiol 2006(101):971–985
Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ (2004) Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557:543–558
Canning BJ, Farmer DG, Mori N (2006) Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 291:R454-463
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Ho CY, Gu Q, Lin YS, Lee LY (2001) Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127:113–124
Widdicombe J (2001) Airway receptors. Respir Physiol 125:3–15
Kajekar R, Proud D, Myers AC, Meeker SN, Undem BJ (1999) Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther 289:682–687
Taylor-Clark TE (2021) Molecular identity, anatomy, gene expression and function of neural crest vs placode-derived nociceptors in the lower airways. Neurosci Lett 742:135505
Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR (1985) Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 2006(101):618–627
Driessen AK, Farrell MJ, Mazzone SB, McGovern AE (2015) The role of the paratrigeminal nucleus in vagal afferent evoked respiratory reflexes: a neuroanatomical and functional study in guinea pigs. Front Physiol 6:378
Fryer AD, Jacoby DB (1992) Function of pulmonary M2 muscarinic receptors in antigen-challenged guinea pigs is restored by heparin and poly-L-glutamate. J Clin Invest 90:2292–2298
Kalia M, Mesulam MM (1980) Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol 193:435–465
Kalia M, Sullivan JM (1982) Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol 211:248–265
Baker DG, McDonald DM, Basbaum CB, Mitchell RA (1986) The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol 246:513–526
Dey RD, Altemus JB, Rodd A, Mayer B, Said SI, Coburn RF (1996) Neurochemical characterization of intrinsic neurons in ferret tracheal plexus. Am J Respir Cell Mol Biol 14:207–216
Fung DC, Beacock DJ, Richardson PS (1992) Vagal control of mucus glycoconjugate secretion into the feline trachea. J Physiol 453:435–447
Dey RD, Satterfield B, Altemus JB (1999) Innervation of tracheal epithelium and smooth muscle by neurons in airway ganglia. Anat Rec 254:166–172
Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD (1997) Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol 273:L93-103
Elbon CL, Jacoby DB, Fryer AD (1995) Pretreatment with an antibody to interleukin-5 prevents loss of pulmonary M2 muscarinic receptor function in antigen-challenged guinea pigs. Am J Respir Cell Mol Biol 12:320–328
Evans CM, Fryer AD, Jacoby DB, Gleich GJ, Costello RW (1997) Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest 100:2254–2262
Fryer AD, Jacoby DB (1991) Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 102:267–271
Fryer AD, Yarkony KA, Jacoby DB (1994) The effect of leukocyte depletion on pulmonary M2 muscarinic receptor function in parainfluenza virus-infected guinea-pigs. Br J Pharmacol 112:588–594
Schultheis AH, Bassett DJ, Fryer AD (1985) Ozone-induced airway hyperresponsiveness and loss of neuronal M2 muscarinic receptor function. J Appl Physiol 1994(76):1088–1097
Costello RW, Evans CM, Yost BL, Belmonte KE, Gleich GJ, Jacoby DB, Fryer AD (1999) Antigen-induced hyperreactivity to histamine: role of the vagus nerves and eosinophils. Am J Physiol 276:L709-714
Wagner EM, Jacoby DB (1985) Methacholine causes reflex bronchoconstriction. J Appl Physiol 1999(86):294–297
Gold WM (1973) Vagally-mediated reflex bronchoconstriction in allergic asthma. Chest 63(Suppl):11S
Sheppard D, Epstein J, Holtzman MJ, Nadel JA, Boushey HA (1982) Dose-dependent inhibition of cold air-induced bronchoconstriction by atropine. J Appl Physiol Respir Environ Exerc Physiol 53:169–174
Simonsson BG, Skoogh BE, Ekström-Jodal B (1972) Exercise-induced airways constriction. Thorax 27:169–180
Oh EJ, Mazzone SB, Canning BJ, Weinreich D (2006) Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol 573:549–564
Mazzone SB, Canning BJ (2013) Autonomic neural control of the airways. Handb Clin Neurol 117:215–228
Ward JK, Barnes PJ, Springall DR, Abelli L, Tadjkarimi S, Yacoub MH, Polak JM, Belvisi MG (1995) Distribution of human i-NANC bronchodilator and nitric oxide-immunoreactive nerves. Am J Respir Cell Mol Biol 13:175–184
Fischer A, Hoffmann B (1996) Nitric oxide synthase in neurons and nerve fibers of lower airways and in vagal sensory ganglia of man: Correlation with neuropeptides. Am J Respir Crit Care Med 154:209–216
Choi JY, Joo NS, Krouse ME, Wu JV, Robbins RC, Ianowski JP, Hanrahan JW, Wine JJ (2007) Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis. J Clin Invest 117:3118–3127
Ellis JL, Undem BJ (1990) Non-adrenergic, non-cholinergic contractions in the electrically field stimulated guinea-pig trachea. Br J Pharmacol 101:875–880
Hsia CC, Hyde DM, Ochs M, Weibel ER (2010) An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181:394–418
Scott GD, Blum ED, Fryer AD, Jacoby DB (2014) Tissue optical clearing, three-dimensional imaging, and computer morphometry in whole mouse lungs and human airways. Am J Respir Cell Mol Biol 51:43–55
Scott GD, Fryer AD, Jacoby DB (2013) Quantifying nerve architecture in murine and human airways using three-dimensional computational mapping. Am J Respir Cell Mol Biol 48:10–16
West PW, Canning BJ, Merlo-Pich E, Woodcock AA, Smith JA (2015) Morphologic Characterization of Nerves in Whole-Mount Airway Biopsies. Am J Respir Crit Care Med 192:30–39
Drake MG, Scott GD, Blum ED, Lebold KM, Nie Z, Lee JJ, Fryer AD, Costello RW, Jacoby DB (2018) Eosinophils increase airway sensory nerve density in mice and in human asthma. Sci Transl Med 10:45
Shapiro CO, Proskocil BJ, Oppegard LJ, Blum ED, Kappel NL, Chang CH, Fryer AD, Jacoby DB, Costello RW, Drake MG (2021) Airway sensory nerve density is increased in chronic cough. Am J Respir Crit Care Med 203:348–355
Larson SD, Schelegle ES, Hyde DM, Plopper CG (2003) The three-dimensional distribution of nerves along the entire intrapulmonary airway tree of the adult rat and the anatomical relationship between nerves and neuroepithelial bodies. Am J Respir Cell Mol Biol 28:592–599
Lamb JP, Sparrow MP (2002) Three-dimensional mapping of sensory innervation with substance p in porcine bronchial mucosa: comparison with human airways. Am J Respir Crit Care Med 166:1269–1281
Yu J, Wang YF, Zhang JW (1985) Structure of slowly adapting pulmonary stretch receptors in the lung periphery. J Appl Physiol 2003(95):385–393
Watanabe N, Horie S, Michael GJ, Spina D, Page CP, Priestley JV (2005) Immunohistochemical localization of vanilloid receptor subtype 1 (TRPV1) in the guinea pig respiratory system. Pulm Pharmacol Ther 18:187–197
Zhao S, Cui J, Wang Y, Xu D, Su Y, Ma J, Gong X, Bai W, Wang J, Cao R (2023) Three-dimensional visualization of the lymphatic, vascular and neural network in rat lung by confocal microscopy. J Mol Histol 5:7–8
Adriaensen D, Timmermans JP, Brouns I, Berthoud HR, Neuhuber WL, Scheuermann DW (1998) Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res 293:395–405
Brouns I, Van Genechten J, Hayashi H, Gajda M, Gomi T, Burnstock G, Timmermans JP, Adriaensen D (2003) Dual sensory innervation of pulmonary neuroepithelial bodies. Am J Respir Cell Mol Biol 28:275–285
Weichselbaum M, Sparrow MP, Hamilton EJ, Thompson PJ, Knight DA (2005) A confocal microscopic study of solitary pulmonary neuroendocrine cells in human airway epithelium. Respir Res 6:115
Fischer A, Canning BJ, Undem BJ, Kummer W (1998) Evidence for an esophageal origin of VIP-IR and NO synthase-IR nerves innervating the guinea pig trachealis: a retrograde neuronal tracing and immunohistochemical analysis. J Comp Neurol 394:326–334
Xie Q, Miki T, Itoh M, Takeuchi Y (1998) Brainstem projections of sensory fibers of the lung: a horseradish peroxidase and c-fos-like immunohistochemical study in the rat. Okajimas Folia Anat Jpn 75:119–129
Dey RD, Altemus JB, Zervos I, Hoffpauir J (1985) Origin and colocalization of CGRP- and SP-reactive nerves in cat airway epithelium. J Appl Physiol 1990(68):770–778
Su Y, Barr J, Jaquish A, Xu J, Verheyden JM, Sun X (2022) Identification of lung innervating sensory neurons and their target specificity. Am J Physiol Lung Cell Mol Physiol 322:L50-l63
Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV (2006) Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 141:1533–1543
Kim SH, Patil MJ, Hadley SH, Bahia PK, Butler SG, Madaram M, Taylor-Clark TE (2022) Mapping of the sensory innervation of the mouse lung by specific vagal and dorsal root ganglion neuronal subsets. eNeuro 9:7
Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A (2017) Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541:176–181
Pincus AB, Huang SJ, Lebold KM, De La Torre U, Proskocil BJ, Drake MG, Nakai H, Fryer AD, Jacoby DB (2022) Multicolor labeling of airway neurons and analysis of parasympathetic heterogeneity. Sci Rep 12:5006
Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450:56–62
Aven L, Ai X (2013) Mechanisms of respiratory innervation during embryonic development. Organogenesis 9:194–198
Sparrow MP, Weichselbaum M, McCray PB (1999) Development of the innervation and airway smooth muscle in human fetal lung. Am J Respir Cell Mol Biol 20:550–560
Tollet J, Everett AW, Sparrow MP (2001) Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn 221:48–60
Pan J, Yeger H, Cutz E (2004) Innervation of pulmonary neuroendocrine cells and neuroepithelial bodies in developing rabbit lung. J Histochem Cytochem 52:379–389
Olsen CR, Colebatch HJH, Mebel PE, Nadel JA, Staub NC (1965) Motor control of pulmonary airways studied by nerve stimulation. J Appl Physiol 20:202–208
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8:1263–1268
Pincus AB, Adhikary S, Lebold KM, Fryer AD, Jacoby DB (2020) Optogenetic control of airway cholinergic neurons in vivo. Am J Respir Cell Mol Biol 62:423–429
Pierce ABP, Proskocil-Chen AB, Fryer BJ, Jacoby AD, Drake DB, Optogenetics MG (2023) shines light on nerve-mediated airway hyperreactivity. Am J Resp Crit Care Med 207:6165
Tränkner D, Hahne N, Sugino K, Hoon MA, Zuker C (2014) Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A 111:11515–11520
Prescott SL, Umans BD, Williams EK, Brust RD, Liberles SD (2020) An airway protection program revealed by sweeping genetic control of vagal afferents. Cell 181:574-589.e514
Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD (2015) Vagal sensory neuron subtypes that differentially control breathing. Cell 161:622–633
Patil MJ, Meeker S, Bautista D, Dong X, Undem BJ (2019) Sphingosine-1-phosphate activates mouse vagal airway afferent C-fibres via S1PR3 receptors. J Physiol 597:2007–2019
Chung KF, McGarvey L, Mazzone SB (2013) Chronic cough as a neuropathic disorder. Lancet Respir Med 1:414–422
Mutoh T, Bonham AC, Joad JP (2000) Substance P in the nucleus of the solitary tract augments bronchopulmonary C fiber reflex output. Am J Physiol Regul Integr Comp Physiol 279:R1215-1223
Moreaux B, Nemmar A, Vincke G, Halloy D, Beerens D, Advenier C, Gustin P (2000) Role of substance P and tachykinin receptor antagonists in citric acid-induced cough in pigs. Eur J Pharmacol 408:305–312
Fahy JV, Wong HH, Geppetti P, Reis JM, Harris SC, Maclean DB, Nadel JA, Boushey HA (1995) Effect of an NK1 receptor antagonist (CP-99,994) on hypertonic saline-induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med 152:879–884
Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, Lerner EA (2017) Substance P activates Mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol 140(447–453):e443
Smith JA, Harle A, Dockry R, Holt K, Russell P, Molassiotis A, Yorke J, Robinson R, Birrell MA, Belvisi MG, Blackhall F (2021) Aprepitant for cough in lung cancer. a randomized placebo-controlled trial and mechanistic insights. Am J Respir Crit Care Med 203:737–745
Noronha V, Bhattacharjee A, Patil VM, Joshi A, Menon N, Shah S, Kannan S, Mukadam SA, Maske K, Ishi S, Prabhash K (2020) Aprepitant for cough suppression in advanced lung cancer: a randomized trial. Chest 157:1647–1655
Smith J, Allman D, Badri H, Miller R, Morris J, Satia I, Wood A, M KT, (2020) The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO-1). Chest 157:111–118
Brouns I, Adriaensen D, Burnstock G, Timmermans JP (2000) Intraepithelial vagal sensory nerve terminals in rat pulmonary neuroepithelial bodies express P2X(3) receptors. Am J Respir Cell Mol Biol 23:52–61
Wang L, Feng D, Yan H, Wang Z, Pei L (2014) Comparative analysis of P2X1, P2X2, P2X3, and P2X4 receptor subunits in rat nodose ganglion neurons. PLoS ONE 9:e96699
Belvisi MG, Smith JA (2017) ATP and cough reflex hypersensitivity: a confusion of goals? Eur Respir J 50:49
Turner RD, Birring SS (2019) Chronic cough: ATP, afferent pathways and hypersensitivity. Eur Respir J 54:78
Fowles HE, Rowland T, Wright C, Morice A (2017) Tussive challenge with ATP and AMP: does it reveal cough hypersensitivity? Eur Respir J 49:78
Morice AH, Kitt MM, Ford AP, Tershakovec AM, Wu WC, Brindle K, Thompson R, Thackray-Nocera S, Wright C (2019) The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J 54:78
Smith JA, Kitt MM, Butera P, Smith SA, Li Y, Xu ZJ, Holt K, Sen S, Sher MR, Ford AP (2020) Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J 55:789
Smith JA, Kitt MM, Morice AH, Birring SS, McGarvey LP, Sher MR, Li YP, Wu WC, Xu ZJ, Muccino DR, Ford AP (2020) Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med 8:775–785
McGarvey LP, Birring SS, Morice AH, Dicpinigaitis PV, Pavord ID, Schelfhout J, Nguyen AM, Li Q, Tzontcheva A, Iskold B, Green SA, Rosa C, Muccino DR, Smith JA (2022) Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 399:909–923
Morice A, Smith JA, McGarvey L, Birring SS, Parker SM, Turner A, Hummel T, Gashaw I, Fels L, Klein S, Francke K, Friedrich C (2021) Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J 58:1–75
McGarvey L, Sher M, Shvarts YG, Lu S, Wu WC, Xu P, Schelfhout J, La Rosa C, Nguyen AM, Reyfman PA, Afzal AS (2023) The Efficacy and safety of Gefapixant in a phase 3b trial of patients with recent-onset chronic cough. Lung 201:111–118
Dicpinigaitis PV, Morice AH, Smith JA, Sher MR, Vaezi M, Guilleminault L, Niimi A, Gude K, Krahn U, Saarinen R, Pires PV, Wosnitza M, McGarvey L (2023) Efficacy and safety of eliapixant in refractory chronic cough: the randomized, placebo-controlled phase 2b PAGANINI study. Lung 201:255–266
Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC Jr, Lambrecht BN (2007) Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13:913–919
Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow JC, Idzko M (2010) Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181:928–934
Mizeepc BJ, Fryer AD, Jacoby DB, Drake MD (2023) Airway eosinophils modulate purinergic receptor P2X3 expression. Am J Res Crit Med 207:6167
Bonvini SJ, Birrell MA, Dubuis E, Adcock JJ, Wortley MA, Flajolet P, Bradding P, Belvisi MG (2020) Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma. Eur Respir J 56:48
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A (2005) The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15:929–934
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Collier JG, Fuller RW (1984) Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol 81:113–117
Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH (2009) TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med 180:1042–1047
Guan M, Ying S, Wang Y (2021) Increased expression of transient receptor potential channels and neurogenic factors associates with cough severity in a guinea pig model. BMC Pulm Med 21:187
Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF (2004) Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 170:1276–1280
Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, Compton SJ, Morice AH (2005) Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res 31:295–306
Lieu TM, Myers AC, Meeker S, Undem BJ (2012) TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 302:L941-948
Zaccone EJ, Lieu T, Muroi Y, Potenzieri C, Undem BE, Gao P, Han L, Canning BJ, Undem BJ (2016) Parainfluenza 3-Induced Cough Hypersensitivity in the Guinea Pig Airways. PLoS ONE 11:e0155526
Zhang G, Lin RL, Wiggers M, Snow DM, Lee LY (2008) Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol 586:5771–5786
Khalid S, Murdoch R, Newlands A, Smart K, Kelsall A, Holt K, Dockry R, Woodcock A, Smith JA (2014) Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol 134:56–62
Belvisi MG, Birrell MA, Wortley MA, Maher SA, Satia I, Badri H, Holt K, Round P, McGarvey L, Ford J, Smith JA (2017) XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am J Respir Crit Care Med 196:1255–1263
Ludbrook VJ, Hanrott KE, Kreindler JL, Marks-Konczalik JE, Bird NP, Hewens DA, Beerahee M, Behm DJ, Morice A, McGarvey L, Parker SM, Birring SS, Smith J (2021) Adaptive study design to assess effect of TRPV4 inhibition in patients with chronic cough. ERJ Open Res 7:00269–02021
Busse WW (2010) The relationship of airway hyperresponsiveness and airway inflammation: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138:4s–10s
Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB (2006) Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest 116:228–236
Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME (2005) The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol 175:5341–5350
Wu YC, Moon HG, Bindokas VP, Phillips EH, Park GY, Lee SS (2023) Multiresolution 3D Optical Mapping of Immune Cell Infiltrates in Mouse Asthmatic Lung. Am J Respir Cell Mol Biol 69:13–21
Lebold KM, Drake MG, Hales-Beck LB, Fryer AD, Jacoby DB (2020) IL-5 exposure in utero increases lung nerve density and airway reactivity in adult offspring. Am J Respir Cell Mol Biol 62:493–502
Lebold KM, Drake MG, Pincus AB, Pierce AB, Fryer AD, Jacoby DB (2022) Unique allergic asthma phenotypes in offspring of house dust mite-exposed mice. Am J Respir Cell Mol Biol 67:89–98
Nie Z, Jacoby DB, Fryer AD (2009) Etanercept prevents airway hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. Br J Pharmacol 156:201–210
Verbout NG, Jacoby DB, Gleich GJ, Fryer AD (2009) Atropine-enhanced, antigen challenge-induced airway hyperreactivity in guinea pigs is mediated by eosinophils and nerve growth factor. Am J Physiol Lung Cell Mol Physiol 297:L228-237
Funding
HL155623 (Matthew G. Drake), HL121254 (Matthew G. Drake).
Author information
Authors and Affiliations
Contributions
JK, UD, EM, and MD wrote the main manuscript text. UD prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
Dr. Drake has received honorarium from Merck, Bellus, Astra Zeneca, and Chiesi.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kornfield, J., De La Torre, U., Mize, E. et al. Illuminating Airway Nerve Structure and Function in Chronic Cough. Lung 201, 499–509 (2023). https://doi.org/10.1007/s00408-023-00659-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-023-00659-x