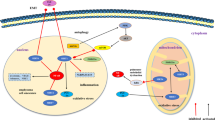
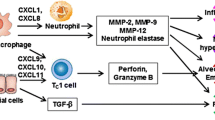
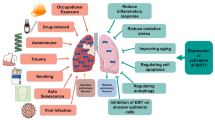
Abstract
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease characterized by irreversible airflow obstruction and lung function decline. It is well established that COPD represents a major cause of morbidity and mortality globally. Due to the substantial economic and social burdens associated with COPD, it is necessary to discover new targets and develop novel beneficial therapies. Although the pathogenesis of COPD is complex and remains to be robustly elucidated, numerous studies have shown that oxidative stress, inflammatory responses, cell apoptosis, autophagy, and aging are involved in the pathogenesis of COPD. Sirtuin 1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase belonging to the silent information regulator 2 (Sir2) family. Multiple studies have indicated that SIRT1 plays an important role in oxidative stress, apoptosis, inflammation, autophagy, and cellular senescence, which contributes to the pathogenesis and development of COPD. This review aimed to discuss the functions and mechanisms of SIRT1 in the progression of COPD and concluded that SIRT1 activation might be a potential therapeutic strategy for COPD.

Similar content being viewed by others
References
Vogelmeier C, Criner G, Martinez F, Anzueto A, Barnes P, Bourbeau J et al (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Critic Care Med 195(5):557–582. https://doi.org/10.1164/rccm.201701-0218PP
Hogea S, Tudorache E, Fildan A, Fira-Mladinescu O, Marc M, Oancea C (2020) Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J 14(3):183–197. https://doi.org/10.1111/crj.13129
Christenson SA, Smith BM, Bafadhel M, Putcha N (2022) Chronic obstructive pulmonary disease. Lancet 399(10342):2227–2242. https://doi.org/10.1016/s0140-6736(22)00470-6
Fu YS, Kang N, Yu Y, Mi Y, Guo J, Wu J et al (2022) Polyphenols, flavonoids and inflammasomes: the role of cigarette smoke in COPD. Eur Respir Rev. https://doi.org/10.1183/16000617.0028-2022
Lu Z, Coll P, Maitre B, Epaud R, Lanone S (2022) Air pollution as an early determinant of COPD. Eur Respir Rev. https://doi.org/10.1183/16000617.0059-2022
Martinez CH, Han MK (2012) Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am 96(4):713–727. https://doi.org/10.1016/j.mcna.2012.02.007
Eapen M, Myers S, Walters E, Sohal S (2017) Airway inflammation in chronic obstructive pulmonary disease (COPD): a true paradox. Expert Rev Respir Med 11(10):827–839. https://doi.org/10.1080/17476348.2017.1360769
Brandsma CA, Van den Berge M, Hackett TL, Brusselle G, Timens W (2020) Recent advances in chronic obstructive pulmonary disease pathogenesis: from disease mechanisms to precision medicine. J Pathol 250(5):624–635. https://doi.org/10.1002/path.5364
Ruaro B, Salton F, Braga L, Wade B, Confalonieri P, Volpe MC et al (2021) The history and mystery of alveolar epithelial type II cells: focus on their physiologic and pathologic role in lung. Int J Mol Sci. https://doi.org/10.3390/ijms22052566
Ferrera MC, Labaki WW, Han MK (2021) Advances in chronic obstructive pulmonary disease. Annu Rev Med 72:119–134. https://doi.org/10.1146/annurev-med-080919-112707
Chan S, Selemidis S, Bozinovski S, Vlahos R (2019) Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies. Pharmacol Ther 198:160–188. https://doi.org/10.1016/j.pharmthera.2019.02.013
Lv Y, Lin S, Peng F (2017) SIRT1 gene polymorphisms and risk of lung cancer. Cancer Manage Res 9:381–386. https://doi.org/10.2147/cmar.S142677
Huang C, Jiang S, Gao S, Wang Y, Cai X, Fang J et al (2022) Sirtuins: research advances on the therapeutic role in acute kidney injury. Phytomedicine 101:154122. https://doi.org/10.1016/j.phymed.2022.154122
Khawar MB, Sohail AM, Li W (2022) SIRT1: a key player in male reproduction. Life. https://doi.org/10.3390/life12020318
Yang Y, Zhang S, Guan J, Jiang Y, Zhang J, Luo L et al (2022) SIRT1 attenuates neuroinflammation by deacetylating HSPA4 in a mouse model of Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis 1868(5):166365. https://doi.org/10.1016/j.bbadis.2022.166365
Shen P, Deng X, Chen Z, Ba X, Qin K, Huang Y et al (2021) SIRT1: a potential therapeutic target in autoimmune diseases. Front Immunol 12:779177. https://doi.org/10.3389/fimmu.2021.779177
Voelter-Mahlknecht S, Mahlknecht U (2006) Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med 17(1):59–67
Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J et al (2020) SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol 22(10):1170–1179. https://doi.org/10.1038/s41556-020-00579-5
Giblin W, Skinner M, Lombard D (2014) Sirtuins: guardians of mammalian healthspan. Trends Genet 30(7):271–286. https://doi.org/10.1016/j.tig.2014.04.007
Frye R (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273(2):793–798. https://doi.org/10.1006/bbrc.2000.3000
Finkel T, Deng C, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460(7255):587–591. https://doi.org/10.1038/nature08197
Kabiljo J, Murko C, Pusch O, Zupkovitz G (2019) Spatio-temporal expression profile of sirtuins during aging of the annual fish Nothobranchius furzeri. Gene Expr Patterns 33:11–19. https://doi.org/10.1016/j.gep.2019.05.001
Haigis M, Guarente L (2006) Mammalian sirtuins–Emerging roles in physiology, aging, and calorie restriction. Genes Dev 20(21):2913–2921. https://doi.org/10.1101/gad.1467506
Tang BL (2016) Sirt1 and the mitochondria. Mol Cells 39(2):87–95. https://doi.org/10.14348/molcells.2016.2318
Wang Y, Yang J, Hong T, Chen X, Cui L (2019) SIRT2: controversy and multiple roles in disease and physiology. Ageing Res Rev 55:100961. https://doi.org/10.1016/j.arr.2019.100961
Zhang XY, Li W, Zhang JR, Li CY, Zhang J, Lv XJ (2022) Roles of sirtuin family members in chronic obstructive pulmonary disease. Respir Res 23(1):66. https://doi.org/10.1186/s12931-022-01986-y
Korytina GF, Akhmadishina LZ, Aznabaeva YG, Kochetova OV, Zagidullin NS, Kzhyshkowska JG et al (2019) Associations of the NRF2/KEAP1 pathway and antioxidant defense gene polymorphisms with chronic obstructive pulmonary disease. Gene 692:102–112. https://doi.org/10.1016/j.gene.2018.12.061
Bakke PS, Zhu G, Gulsvik A, Kong X, Agusti AG, Calverley PM et al (2011) Candidate genes for COPD in two large data sets. Eur Respir J 37(2):255–263. https://doi.org/10.1183/09031936.00091709
Zhang M, Zhang Y, Roth M, Zhang L, Shi R, Yang X et al (2020) Sirtuin 3 inhibits airway epithelial mitochondrial oxidative stress in cigarette smoke-induced COPD. Oxid Med Cell Longev 2020:7582980. https://doi.org/10.1155/2020/7582980
Chen Y, Wang H, Luo G, Dai X (2014) SIRT4 inhibits cigarette smoke extracts-induced mononuclear cell adhesion to human pulmonary microvascular endothelial cells via regulating NF-κB activity. Toxicol Lett 226(3):320–327. https://doi.org/10.1016/j.toxlet.2014.02.022
Wang Y, Zhu Y, Xing S, Ma P, Lin D (2015) SIRT5 prevents cigarette smoke extract-induced apoptosis in lung epithelial cells via deacetylation of FOXO3. Cell Stress Chaperones 20(5):805–810. https://doi.org/10.1007/s12192-015-0599-7
Kato R, Mizuno S, Kadowaki M, Shiozaki K, Akai M, Nakagawa K et al (2016) Sirt1 expression is associated with CD31 expression in blood cells from patients with chronic obstructive pulmonary disease. Respir Res 17(1):139. https://doi.org/10.1186/s12931-016-0452-2
Yang S, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I (2007) Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292(2):L567–L576. https://doi.org/10.1152/ajplung.00308.2006
Iqbal IK, Bajeli S, Sahu S, Bhat SA, Kumar A (2021) Hydrogen sulfide-induced GAPDH sulfhydration disrupts the CCAR2-SIRT1 interaction to initiate autophagy. Autophagy 17(11):3511–3529. https://doi.org/10.1080/15548627.2021.1876342
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE et al (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105(9):3374–3379. https://doi.org/10.1073/pnas.0712145105
He B, Chen Q, Zhou D, Wang L, Liu Z (2019) Role of reciprocal interaction between autophagy and endoplasmic reticulum stress in apoptosis of human bronchial epithelial cells induced by cigarette smoke extract. IUBMB Life 71(1):66–80. https://doi.org/10.1002/iub.1937
Wang S, He N, Xing H, Sun Y, Ding J, Liu L (2020) Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1α/NF-κB signaling axis. J Recept Signal Transduct Res 40(4):388–394. https://doi.org/10.1080/10799893.2020.1738483
Yao H, Sundar I, Ahmad T, Lerner C, Gerloff J, Friedman A et al (2014) SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 306(9):L816–L828. https://doi.org/10.1152/ajplung.00323.2013
Guan R, Wang J, Cai Z, Li Z, Wang L, Li Y et al (2020) Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol 28:101356. https://doi.org/10.1016/j.redox.2019.101356
Gu C, Li Y, Xu WL, Yan JP, Xia YJ, Ma YY et al (2015) Sirtuin 1 activator SRT1720 protects against lung injury via reduction of type II alveolar epithelial cells apoptosis in emphysema. COPD 12(4):444–452. https://doi.org/10.3109/15412555.2014.974740
Ma N, Deng TT, Wang Q, Luo ZL, Zhu CF, Qiu JF et al (2019) Erythromycin regulates cigarette smoke-induced proinflammatory mediator release through sirtuin 1-nuclear factor κB axis in macrophages and mice lungs. Pathobiology 86(5–6):237–247. https://doi.org/10.1159/000500628
Peng Z, Zhang W, Qiao J, He B (2018) Melatonin attenuates airway inflammation via SIRT1 dependent inhibition of NLRP3 inflammasome and IL-1β in rats with COPD. Int Immunopharmacol 62:23–28. https://doi.org/10.1016/j.intimp.2018.06.033
Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW et al (2010) Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys 500(2):203–209. https://doi.org/10.1016/j.abb.2010.05.013
Baker JR, Vuppusetty C, Colley T, Papaioannou AI, Fenwick P, Donnelly L et al (2016) Oxidative stress dependent microRNA-34a activation via PI3Kα reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci Rep 6:35871. https://doi.org/10.1038/srep35871
Baker JR, Vuppusetty C, Colley T, Hassibi S, Fenwick PS, Donnelly LE et al (2019) MicroRNA-570 is a novel regulator of cellular senescence and inflammaging. Faseb j 33(2):1605–1616. https://doi.org/10.1096/fj.201800965R
Wu H, Ma H, Wang L, Zhang H, Lu L, Xiao T et al (2022) Regulation of lung epithelial cell senescence in smoking-induced COPD/emphysema by microR-125a-5p via Sp1 mediation of SIRT1/HIF-1a. Int J Biol Sci 18(2):661–674. https://doi.org/10.7150/ijbs.65861
Forman HJ, Zhang H (2021) Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20(9):689–709. https://doi.org/10.1038/s41573-021-00233-1
Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS et al (2022) Oxidative stress in human pathology and aging molecular mechanisms and perspectives. Cells. https://doi.org/10.3390/cells11030552
Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI (2021) Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur J Med Chem 209:112891. https://doi.org/10.1016/j.ejmech.2020.112891
Caliri AW, Tommasi S, Besaratinia A (2021) Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res 787:108365. https://doi.org/10.1016/j.mrrev.2021.108365
Barnes PJ (2022) Oxidative stress in chronic obstructive pulmonary disease. Antioxidants. https://doi.org/10.3390/antiox11050965
Mumby S, Adcock IM (2022) Recent evidence from omic analysis for redox signalling and mitochondrial oxidative stress in COPD. J Inflamm 19(1):10. https://doi.org/10.1186/s12950-022-00308-9
Taniguchi A, Tsuge M, Miyahara N, Tsukahara H (2021) Reactive oxygen species and antioxidative defense in chronic obstructive pulmonary disease. Antioxidants. https://doi.org/10.3390/antiox10101537
Hikichi M, Mizumura K, Maruoka S, Gon Y (2019) Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J Thorac Dis 11(Suppl 17):S2129-s40. https://doi.org/10.21037/jtd.2019.10.43
Song Q, Zhou ZJ, Cai S, Chen Y, Chen P (2022) Oxidative stress links the tumour suppressor p53 with cell apoptosis induced by cigarette smoke. Int J Environ Health Res 32(8):1745–1755. https://doi.org/10.1080/09603123.2021.1910211
de Groot L, van der Veen T, Martinez F, Hamann J, Lutter R, Melgert B (2019) Oxidative stress and macrophages: driving forces behind exacerbations of asthma and chronic obstructive pulmonary disease? Am J Physiol Lung Cell Mol Physiol 316(2):L369–L384. https://doi.org/10.1152/ajplung.00456.2018
Wiegman C, Michaeloudes C, Haji G, Narang P, Clarke C, Russell K et al (2015) Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 136(3):769–780. https://doi.org/10.1016/j.jaci.2015.01.046
Jiang Y, Wang X, Hu D (2017) Mitochondrial alterations during oxidative stress in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 12:1153–1162. https://doi.org/10.2147/copd.S130168
Ornatowski W, Lu Q, Yegambaram M, Garcia AE, Zemskov EA, Maltepe E et al (2020) Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol 36:101679. https://doi.org/10.1016/j.redox.2020.101679
Aggarwal T, Wadhwa R, Rohil V, Maurya P (2018) Biomarkers of oxidative stress and protein-protein interaction in chronic obstructive pulmonary disease. Arch Physiol Biochem 124(3):226–231. https://doi.org/10.1080/13813455.2017.1387796
Zinellu E, Zinellu A, Fois A, Fois S, Piras B, Carru C et al (2020) Reliability and usefulness of different biomarkers of oxidative stress in chronic obstructive pulmonary disease. Oxid Med Cell Longev 2020:4982324. https://doi.org/10.1155/2020/4982324
Zinellu E, Zinellu A, Fois A, Carru C, Pirina P (2016) Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: a systematic review. Respir Res 17(1):150. https://doi.org/10.1186/s12931-016-0471-z
Nucera F, Mumby S, Paudel KR, Dharwal V, Di Stefano A, Casolaro V et al (2022) Role of oxidative stress in the pathogenesis of COPD. Minerva Med 113(3):370–404. https://doi.org/10.23736/s0026-4806.22.07972-1
Li L, Wang H, Zhao S, Zhao Y, Chen Y, Zhang J et al (2022) Paeoniflorin ameliorates lipopolysaccharide-induced acute liver injury by inhibiting oxidative stress and inflammation via SIRT1/FOXO1a/SOD2 signaling in rats. Phytother Res. https://doi.org/10.1002/ptr.7471
Zhang Y, Li T, Pan M, Wang W, Huang W, Yuan Y et al (2022) SIRT1 prevents cigarette smoking-induced lung fibroblasts activation by regulating mitochondrial oxidative stress and lipid metabolism. J Transl Med 20(1):222. https://doi.org/10.1186/s12967-022-03408-5
Wang AJ, Tang Y, Zhang J, Wang BJ, Xiao M, Lu G et al (2022) Cardiac SIRT1 ameliorates doxorubicin-induced cardiotoxicity by targeting sestrin 2. Redox Biol 52:102310. https://doi.org/10.1016/j.redox.2022.102310
Feng K, Chen Z, Pengcheng L, Zhang S, Wang X (2019) Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J Cell Physiol 234(10):18192–18205. https://doi.org/10.1002/jcp.28452
Yanagisawa S, Papaioannou A, Papaporfyriou A, Baker J, Vuppusetty C, Loukides S et al (2017) Decreased serum sirtuin-1 in COPD. Chest 152(2):343–352. https://doi.org/10.1016/j.chest.2017.05.004
Conti V, Corbi G, Manzo V, Malangone P, Vitale C, Maglio A et al (2018) SIRT1 activity in peripheral blood mononuclear cells correlates with altered lung function in patients with chronic obstructive pulmonary disease. Oxid Med Cell Longev 2018:9391261. https://doi.org/10.1155/2018/9391261
Lee KH, Jeong J, Koo YJ, Jang AH, Lee CH, Yoo CG (2017) Exogenous neutrophil elastase enters bronchial epithelial cells and suppresses cigarette smoke extract-induced heme oxygenase-1 by cleaving sirtuin 1. J Biol Chem 292(28):11970–11979. https://doi.org/10.1074/jbc.M116.771089
Zhao X, Wu Y (2021) Correlations of Silent Information Regulator of Transcription 1 (SIRT1) expression, inflammatory factors, and oxidative stress with pulmonary function in patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD). Med Sci Monit 27:e929046. https://doi.org/10.12659/msm.929046
Hwang JW, Yao H, Caito S, Sundar IK, Rahman I (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110. https://doi.org/10.1016/j.freeradbiomed.2013.03.015
Conti V, Corbi G, Manzo V, Pelaia G, Filippelli A, Vatrella A (2015) Sirtuin 1 and aging theory for chronic obstructive pulmonary disease. Anal Cell Pathol 2015:897327. https://doi.org/10.1155/2015/897327
Hwang JW, Rajendrasozhan S, Yao H, Chung S, Sundar IK, Huyck HL et al (2011) FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol 187(2):987–998. https://doi.org/10.4049/jimmunol.1001861
Di Vincenzo S, Heijink IH, Noordhoek JA, Cipollina C, Siena L, Bruno A et al (2018) SIRT1/FoxO3 axis alteration leads to aberrant immune responses in bronchial epithelial cells. J Cell Mol Med 22(4):2272–2282. https://doi.org/10.1111/jcmm.13509
Dutta RK, Chinnapaiyan S, Rasmussen L, Raju SV, Unwalla HJ (2019) A neutralizing aptamer to TGFBR2 and miR-145 antagonism rescue cigarette smoke- and TGF-β-mediated CFTR expression. Mol Ther 27(2):442–455. https://doi.org/10.1016/j.ymthe.2018.11.017
Michaeloudes C, Sukkar M, Khorasani N, Bhavsar P, Chung K (2011) TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300(2):L295-304. https://doi.org/10.1152/ajplung.00134.2010
Casalena G, Daehn I, Bottinger E (2012) Transforming growth factor-beta, bioenergetics, and mitochondria in renal disease. Semin Nephrol 32(3):295–303. https://doi.org/10.1016/j.semnephrol.2012.04.009
Krstic J, Trivanovic D, Mojsilovic S, Santibanez JF (2015) Transforming growth factor-beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev 2015:654594. https://doi.org/10.1155/2015/654594
Garcia-Vizcaino EM, Liarte S, Alonso-Romero JL, Nicolas FJ (2017) Sirt1 interaction with active Smad2 modulates transforming growth factor-beta regulated transcription. Cell Commun Signal 15(1):50. https://doi.org/10.1186/s12964-017-0205-y
Ren Y, Zhang Y, Fan L, Jiao Q, Wang Y, Wang Q (2019) The cullin4A is up-regulated in chronic obstructive pulmonary disease patient and contributes to epithelial-mesenchymal transition in small airway epithelium. Respir Res 20(1):84. https://doi.org/10.1186/s12931-019-1048-4
Lee HW, Jose CC, Cuddapah S (2021) Epithelial-mesenchymal transition: insights into nickel-induced lung diseases. Semin Cancer Biol 76:99–109. https://doi.org/10.1016/j.semcancer.2021.05.020
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592. https://doi.org/10.1002/cbin.11137
Gong J, Zhao H, Liu T, Li L, Cheng E, Zhi S et al (2019) Cigarette smoke reduces fatty acid catabolism, leading to apoptosis in lung endothelial cells: implication for pathogenesis of COPD. Front Pharmacol 10:941. https://doi.org/10.3389/fphar.2019.00941
Sauler M, Bazan IS, Lee PJ (2019) Cell death in the lung: the apoptosis-necroptosis axis. Annu Rev Physiol 81:375–402. https://doi.org/10.1146/annurev-physiol-020518-114320
Vijayan VK (2013) Chronic obstructive pulmonary disease. Indian J Med Res 137(2):251–269
D’Agostini F, Balansky R, Izzotti A, Lubet R, Kelloff G, De Flora S (2001) Modulation of apoptosis by cigarette smoke and cancer chemopreventive agents in the respiratory tract of rats. Carcinogenesis 22(3):375–380. https://doi.org/10.1093/carcin/22.3.375
Murray L, Dunmore R, Camelo A, Da Silva C, Gustavsson M, Habiel D et al (2017) Acute cigarette smoke exposure activates apoptotic and inflammatory programs but a second stimulus is required to induce epithelial to mesenchymal transition in COPD epithelium. Respir Res 18(1):82. https://doi.org/10.1186/s12931-017-0565-2
Segura-Valdez L, Pardo A, Gaxiola M, Uhal B, Becerril C, Selman M (2000) Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 117(3):684–694. https://doi.org/10.1378/chest.117.3.684
Park J, Ryter S, Choi A (2007) Functional significance of apoptosis in chronic obstructive pulmonary disease. COPD 4(4):347–353. https://doi.org/10.1080/15412550701603775
Henson P, Vandivier R, Douglas I (2006) Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc 3(8):713–717. https://doi.org/10.1513/pats.200605-104SF
Liu H, Ma L, Wu J, Wang K, Chen X (2009) Apoptosis of alveolar wall cells in chronic obstructive pulmonary disease patients with pulmonary emphysema is involved in emphysematous changes. J Huazhong Univ Sci Technolog Med Sci 29(4):466–469. https://doi.org/10.1007/s11596-009-0415-7
Song Q, Chen P, Liu XM (2021) The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir Res 22(1):39. https://doi.org/10.1186/s12931-021-01630-1
Imai K, Mercer B, Schulman L, Sonett J, D’Armiento J (2005) Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J 25(2):250–258. https://doi.org/10.1183/09031936.05.00023704
Khanahmadi M, Manafi B, Tayebinia H, Karimi J, Khodadadi I (2020) Downregulation of Sirt1 is correlated to upregulation of p53 and increased apoptosis in epicardial adipose tissue of patients with coronary artery disease. Excli j 19:1387–1398. https://doi.org/10.17179/excli2020-2423
Yu Y, Li L, Yu W, Guan Z (2022) Fluoride exposure suppresses proliferation and enhances endoplasmic reticulum stress and apoptosis pathways in hepatocytes by downregulating sirtuin-1. Biomed Res Int 2022:7380324. https://doi.org/10.1155/2022/7380324
Li M, Li SC, Dou BK, Zou YX, Han HZ, Liu DX et al (2020) Cycloastragenol upregulates SIRT1 expression, attenuates apoptosis and suppresses neuroinflammation after brain ischemia. Acta Pharmacol Sin 41(8):1025–1032. https://doi.org/10.1038/s41401-020-0386-6
Luo G, Jian Z, Zhu Y, Zhu Y, Chen B, Ma R et al (2019) Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int J Mol Med 43(5):2033–2043. https://doi.org/10.3892/ijmm.2019.4125
Qiu D, Song S, Wang Y, Bian Y, Wu M, Wu H et al (2022) NAD(P)H: quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating Sirt1 in diabetic nephropathy. J Transl Med 20(1):44. https://doi.org/10.1186/s12967-021-03197-3
Chen Y, Zhou F, Liu H, Li J, Che H, Shen J et al (2021) SIRT1, a promising regulator of bone homeostasis. Life Sci 269:119041. https://doi.org/10.1016/j.lfs.2021.119041
Yang H, Chen J, Chen Y, Jiang Y, Ge B, Hong L (2021) Sirt1 activation negatively regulates overt apoptosis in Mtb-infected macrophage through Bax. Int Immunopharmacol 91:107283. https://doi.org/10.1016/j.intimp.2020.107283
He B, Zhang W, Qiao J, Peng Z, Chai X (2019) Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can J Physiol Pharmacol 97(5):386–391. https://doi.org/10.1139/cjpp-2018-0529
Zhang L, Guo X, Xie W, Li Y, Ma M, Yuan T et al (2015) Resveratrol exerts an anti-apoptotic effect on human bronchial epithelial cells undergoing cigarette smoke exposure. Mol Med Rep 11(3):1752–1758. https://doi.org/10.3892/mmr.2014.2925
Zhang L, Luo B, Ting Y, He S, Xie L, Sun S (2020) SIRT1 attenuates endoplasmic reticulum stress and apoptosis in rat models of COPD. Growth Factors 38(2):94–104. https://doi.org/10.1080/08977194.2020.1810029
Hernández Borrero LJ, El-Deiry WS (1876) (2021) Tumor suppressor p53: biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer 1:188556. https://doi.org/10.1016/j.bbcan.2021.188556
Mizuno S, Ishizaki T, Kadowaki M, Akai M, Shiozaki K, Iguchi M et al (2017) p53 signaling pathway polymorphisms associated with emphysematous changes in patients with COPD. Chest 152(1):58–69. https://doi.org/10.1016/j.chest.2017.03.012
Lee H, Jung TY, Lim SH, Choi EJ, Lee J, Min DS (2021) Phospholipase D2 is a positive regulator of sirtuin 1 and modulates p53-mediated apoptosis via sirtuin 1. Exp Mol Med 53(9):1287–1297. https://doi.org/10.1038/s12276-021-00659-y
Audrito V, Vaisitti T, Rossi D, Gottardi D, D’Arena G, Laurenti L et al (2011) Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network. Can Res 71(13):4473–4483. https://doi.org/10.1158/0008-5472.Can-10-4452
Rahman S, Islam R (2011) Mammalian Sirt1: insights on its biological functions. Cell Commun Signal 9:11. https://doi.org/10.1186/1478-811x-9-11
Lam E, Francis R, Petkovic M (2006) FOXO transcription factors: key regulators of cell fate. Biochem Soc Trans 34:722–726. https://doi.org/10.1042/bst0340722
Giannakou M, Partridge L (2004) The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol 14(8):408–412. https://doi.org/10.1016/j.tcb.2004.07.006
Brunet A, Sweeney L, Sturgill J, Chua K, Greer P, Lin Y et al (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303(5666):2011–2015. https://doi.org/10.1126/science.1094637
Brightling C, Greening N (2019) Airway inflammation in COPD: progress to precision medicine. Eur Respir J. https://doi.org/10.1183/13993003.00651-2019
Aghasafari P, George U, Pidaparti R (2019) A review of inflammatory mechanism in airway diseases. Inflamm Res 68(1):59–74. https://doi.org/10.1007/s00011-018-1191-2
Zuo L, Wijegunawardana D (2021) Redox role of ROS and inflammation in pulmonary diseases. Adv Exp Med Biol 1304:187–204. https://doi.org/10.1007/978-3-030-68748-9_11
Shyam Prasad Shetty B, Chaya SK, Kumar VS, Mahendra M, Jayaraj BS, Lokesh KS et al (2021) Inflammatory Biomarkers Interleukin 1 Beta (IL-1β) and Tumour Necrosis Factor Alpha (TNF-α) are differentially elevated in tobacco smoke associated copd and biomass smoke associated COPD. Toxics. https://doi.org/10.3390/toxics9040072
Singh S, Verma SK, Kumar S, Ahmad MK, Nischal A, Singh SK et al (2018) Correlation of severity of chronic obstructive pulmonary disease with potential biomarkers. Immunol Lett 196:1–10. https://doi.org/10.1016/j.imlet.2018.01.004
Fermont JM, Masconi KL, Jensen MT, Ferrari R, Di Lorenzo VAP, Marott JM et al (2019) Biomarkers and clinical outcomes in COPD: a systematic review and meta-analysis. Thorax 74(5):439–446. https://doi.org/10.1136/thoraxjnl-2018-211855
Mulvanny A, Pattwell C, Beech A, Southworth T, Singh D (2022) Validation of sputum biomarker immunoassays and cytokine expression profiles in COPD. Biomedicines. https://doi.org/10.3390/biomedicines10081949
Zeng YY, Hu WP, Zuo YH, Wang XR, Zhang J (2019) Altered serum levels of type I collagen turnover indicators accompanied by IL-6 and IL-8 release in stable COPD. Int J Chron Obstruct Pulmon Dis 14:163–168. https://doi.org/10.2147/copd.S188139
Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y et al (2022) Regulation of SIRT1 and its roles in inflammation. Front Immunol 13:831168. https://doi.org/10.3389/fimmu.2022.831168
Sun HJ, Xiong SP, Cao X, Cao L, Zhu MY, Wu ZY et al (2021) Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol 38:101813. https://doi.org/10.1016/j.redox.2020.101813
Labiner HE, Sas KM, Baur JA, Sims CA (2022) SIRT1 deletion increases inflammation and mortality in sepsis. J Trauma Acute Care Surg. https://doi.org/10.1097/ta.0000000000003751
Liu X, Zheng H (2021) Modulation of Sirt1 and FoxO1 on hypothalamic leptin-mediated sympathetic activation and inflammation in diet-induced obese rats. J Am Heart Assoc 10(14):e020667. https://doi.org/10.1161/jaha.120.020667
Shatoor AS, Al HS (2021) Astaxanthin Ameliorates high-fat diet-induced cardiac damage and fibrosis by upregulating and activating SIRT1. Saudi J Biol Sci 28(12):7012–7021. https://doi.org/10.1016/j.sjbs.2021.07.079
Chen M, Chen Z, Huang D, Sun C, Xie J, Chen T et al (2020) Myricetin inhibits TNF-α-induced inflammation in A549 cells via the SIRT1/NF-κB pathway. Pulm Pharmacol Ther 65:102000. https://doi.org/10.1016/j.pupt.2021.102000
Alharbi KS, Fuloria NK, Fuloria S, Rahman SB, Al-Malki WH, Javed Shaikh MA et al (2021) Nuclear factor-kappa B and its role in inflammatory lung disease. Chem Biol Interact 345:109568. https://doi.org/10.1016/j.cbi.2021.109568
Benjamin JT, Plosa EJ, Sucre JM, van der Meer R, Dave S, Gutor S et al (2021) Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD. J Clin Invest. https://doi.org/10.1172/jci139481
Wu H, Miao Y, Shang LQ, Chen RL, Yang SM (2020) MiR-31 aggravates inflammation and apoptosis in COPD rats via activating the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci 24(18):9626–9632. https://doi.org/10.26355/eurrev_202009_23051
Won M, Byun H, Park K, Hur G (2016) Post-translational control of NF-κB signaling by ubiquitination. Arch Pharmacal Res 39(8):1075–1084. https://doi.org/10.1007/s12272-016-0772-2
de Gregorio E, Colell A, Morales A, Marí M (2020) Relevance of SIRT1-NF-κB axis as therapeutic target to ameliorate inflammation in liver disease. Int J Mol Sci. https://doi.org/10.3390/ijms21113858
Mendes KL, Lelis DF, Santos SHS (2017) Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev 38:98–105. https://doi.org/10.1016/j.cytogfr.2017.11.001
Min JH, Kim MG, Kim SM, Park JW, Chun W, Lee HJ et al (2020) 3,4,5-Trihydroxycinnamic acid exerts a protective effect on pulmonary inflammation in an experimental animal model of COPD. Int Immunopharmacol 85:106656. https://doi.org/10.1016/j.intimp.2020.106656
Zhang J, Xu Q, Sun W, Zhou X, Fu D, Mao L (2021) New insights into the role of NLRP3 inflammasome in pathogenesis and treatment of chronic obstructive pulmonary disease. J Inflamm Res 14:4155–4168. https://doi.org/10.2147/jir.S324323
Li Y, Yang X, He Y, Wang W, Zhang J, Zhang W et al (2017) Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology 222(3):552–561. https://doi.org/10.1016/j.imbio.2016.11.002
Tian Q, Xu M, He B (2021) Histidine ameliorates elastase- and lipopolysaccharide-induced lung inflammation by inhibiting the activation of the NLRP3 inflammasome. Acta Biochim Biophys Sin 53(8):1055–1064. https://doi.org/10.1093/abbs/gmab072
Ichimiya T, Yamakawa T, Hirano T, Yokoyama Y, Hayashi Y, Hirayama D et al (2020) Autophagy and autophagy-related diseases: a review. Int J Mol Sci. https://doi.org/10.3390/ijms21238974
Soreng K, Neufeld TP, Simonsen A (2018) Membrane trafficking in autophagy. Int Rev Cell Mol Biol 336:1–92. https://doi.org/10.1016/bs.ircmb.2017.07.001
Morishita H, Kanda Y, Kaizuka T, Chino H, Nakao K, Miki Y et al (2020) Autophagy is required for maturation of surfactant-containing lamellar bodies in the lung and swim bladder. Cell Rep 33(10):108477. https://doi.org/10.1016/j.celrep.2020.108477
Barnes PJ, Baker J, Donnelly LE (2022) Autophagy in asthma and chronic obstructive pulmonary disease. Clin Sci 136(10):733–746. https://doi.org/10.1042/cs20210900
Vishnupriya S, Priya Dharshini LC, Sakthivel KM, Rasmi RR (2020) Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci 260:118308. https://doi.org/10.1016/j.lfs.2020.118308
Lv X, Li K, Hu Z (2020) Chronic obstructive pulmonary disease and autophagy. Adv Exp Med Biol 1207:559–567. https://doi.org/10.1007/978-981-15-4272-5_39
Racanelli AC, Choi AMK, Choi ME (2020) Autophagy in chronic lung disease. Prog Mol Biol Transl Sci 172:135–156. https://doi.org/10.1016/bs.pmbts.2020.02.001
Zhuang H, Li N, Chen S, Shen Y, Zhan W, Xu X et al (2020) Correlation between level of autophagy and frequency of CD8(+) T cells in patients with chronic obstructive pulmonary disease. J Int Med Res 48(9):300060520952638. https://doi.org/10.1177/0300060520952638
Wang G, Zhou H, Strulovici-Barel Y, Al-Hijji M, Ou X, Salit J et al (2017) Role of OSGIN1 in mediating smoking-induced autophagy in the human airway epithelium. Autophagy 13(7):1205–1220. https://doi.org/10.1080/15548627.2017.1301327
Tulen CBM, Wang Y, Beentjes D, Jessen PJJ, Ninaber DK, Reynaert NL et al (2022) Dysregulated mitochondrial metabolism upon cigarette smoke exposure in various human bronchial epithelial cell models. Dis Model Mech. https://doi.org/10.1242/dmm.049247
Liu Y, Xu J, Liu T, Wu J, Zhao J, Wang J et al (2021) FSTL1 aggravates cigarette smoke-induced airway inflammation and airway remodeling by regulating autophagy. BMC Pulm Med 21(1):45. https://doi.org/10.1186/s12890-021-01409-6
Huang HQ, Li N, Li DY, Jing D, Liu ZY, Xu XC et al (2021) Autophagy promotes cigarette smoke-initiated and elastin-driven bronchitis-like airway inflammation in mice. Front Immunol 12:594330. https://doi.org/10.3389/fimmu.2021.594330
Wang L, Xu C, Johansen T, Berger S, Dou Z (2020) SIRT1—A new mammalian substrate of nuclear autophagy. Autophagy. https://doi.org/10.1080/15548627.2020.1860541
Tang F, Ling C (2019) Curcumin ameliorates chronic obstructive pulmonary disease by modulating autophagy and endoplasmic reticulum stress through regulation of SIRT1 in a rat model. J Int Med Res 47(10):4764–4774. https://doi.org/10.1177/0300060519869459
Yuan P, Hu Q, He X, Long Y, Song X, Wu F et al (2020) Laminar flow inhibits the Hippo/YAP pathway via autophagy and SIRT1-mediated deacetylation against atherosclerosis. Cell Death Dis 11(2):141. https://doi.org/10.1038/s41419-020-2343-1
Deng Z, Sun M, Wu J, Fang H, Cai S, An S et al (2021) SIRT1 attenuates sepsis-induced acute kidney injury via Beclin1 deacetylation-mediated autophagy activation. Cell Death Dis 12(2):217. https://doi.org/10.1038/s41419-021-03508-y
Kim JY, Mondaca-Ruff D, Singh S, Wang Y (2022) SIRT1 and autophagy: implications in endocrine disorders. Front Endocrinol 13:930919. https://doi.org/10.3389/fendo.2022.930919
Tao Z, Shi L, Parke J, Zheng L, Gu W, Dong XC et al (2021) Sirt1 coordinates with ERα to regulate autophagy and adiposity. Cell Death Discov 7(1):53. https://doi.org/10.1038/s41420-021-00438-8
Mu N, Lei Y, Wang Y, Wang Y, Duan Q, Ma G et al (2019) Inhibition of SIRT1/2 upregulates HSPA5 acetylation and induces pro-survival autophagy via ATF4-DDIT4-mTORC1 axis in human lung cancer cells. Apoptosis 24(9–10):798–811. https://doi.org/10.1007/s10495-019-01559-3
Choudhury G, MacNee W (2017) Role of inflammation and oxidative stress in the pathology of ageing in COPD: potential therapeutic interventions. COPD 14(1):122–135. https://doi.org/10.1080/15412555.2016.1214948
Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF et al (2013) Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Investig 123(12):5212–5230. https://doi.org/10.1172/jci69636
Kadam A, Jubin T, Roychowdhury R, Begum R (2020) Role of PARP-1 in mitochondrial homeostasis. Biochim Biophys Acta Gen Subj 1864(10):129669. https://doi.org/10.1016/j.bbagen.2020.129669
Kang M, Park S, Park SH, Lee HG, Park JH (2022) A double-edged sword: the two faces of PARylation. Int J Mol Sci. https://doi.org/10.3390/ijms23179826
Walko TD 3rd, Di Caro V, Piganelli J, Billiar TR, Clark RS, Aneja RK (2015) Poly(ADP-ribose) polymerase 1-sirtuin 1 functional interplay regulates LPS-mediated high mobility group box 1 secretion. Mol Med 20(1):612–624. https://doi.org/10.2119/molmed.2014.00156
Wang Y, Sui Y, Niu Y, Liu D, Xu Q, Liu F et al (2022) PBX1-SIRT1 positive feedback loop attenuates ROS-mediated HF-MSC senescence and apoptosis. Stem Cell Rev Rep. https://doi.org/10.1007/s12015-022-10425-w
Dharwal V, Naura AS (2018) PARP-1 inhibition ameliorates elastase induced lung inflammation and emphysema in mice. Biochem Pharmacol 150:24–34. https://doi.org/10.1016/j.bcp.2018.01.027
Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW (2014) Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal 20(3):474–494. https://doi.org/10.1089/ars.2013.5373
Shi J, Yin N, Xuan LL, Yao CS, Meng AM, Hou Q (2012) Vam3, a derivative of resveratrol, attenuates cigarette smoke-induced autophagy. Acta Pharmacol Sin 33(7):888–896. https://doi.org/10.1038/aps.2012.73
Cho SJ, Stout-Delgado HW (2020) Aging and lung disease. Annu Rev Physiol 82:433–459. https://doi.org/10.1146/annurev-physiol-021119-034610
Schneider JL, Rowe JH, Garcia-de-Alba C, Kim CF, Sharpe AH, Haigis MC (2021) The aging lung: physiology, disease, and immunity. Cell 184(8):1990–2019. https://doi.org/10.1016/j.cell.2021.03.005
Barnes PJ, Baker J, Donnelly LE (2019) Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med 200(5):556–564. https://doi.org/10.1164/rccm.201810-1975TR
MacNee W (2016) Is chronic obstructive pulmonary disease an accelerated aging disease? Ann Am Thorac Soc. https://doi.org/10.1513/AnnalsATS.201602-124AW
López-Otín C, Blasco M, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Easter M, Bollenbecker S, Barnes JW, Krick S (2020) Targeting aging pathways in chronic obstructive pulmonary disease. Int J Mol Sci. https://doi.org/10.3390/ijms21186924
Hamsanathan S, Alder JK, Sellares J, Rojas M, Gurkar AU, Mora AL (2019) Cellular senescence: the trojan horse in chronic lung diseases. Am J Respir Cell Mol Biol 61(1):21–30. https://doi.org/10.1165/rcmb.2018-0410TR
Woldhuis RR, Heijink IH, van den Berge M, Timens W, Oliver BGG, de Vries M et al (2021) COPD-derived fibroblasts secrete higher levels of senescence-associated secretory phenotype proteins. Thorax 76(5):508–511. https://doi.org/10.1136/thoraxjnl-2020-215114
Aghali A, Koloko Ngassie ML, Pabelick CM, Prakash YS (2022) Cellular senescence in aging lungs and diseases. Cells. https://doi.org/10.3390/cells11111781
Cottage CT, Peterson N, Kearley J, Berlin A, Xiong X, Huntley A et al (2019) Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun Biol 2:307. https://doi.org/10.1038/s42003-019-0532-1
Guan R, Cai Z, Wang J, Ding M, Li Z, Xu J et al (2019) Hydrogen sulfide attenuates mitochondrial dysfunction-induced cellular senescence and apoptosis in alveolar epithelial cells by upregulating sirtuin 1. Aging 11(24):11844–11864. https://doi.org/10.18632/aging.102454
Yan P, Li Z, Xiong J, Geng Z, Wei W, Zhang Y et al (2021) LARP7 ameliorates cellular senescence and aging by allosterically enhancing SIRT1 deacetylase activity. Cell Rep 37(8):110038. https://doi.org/10.1016/j.celrep.2021.110038
Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG et al (2013) Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells 31(12):2813–2826. https://doi.org/10.1002/stem.1488
Ahmad T, Sundar IK, Tormos AM, Lerner CA, Gerloff J, Yao H et al (2017) Shelterin telomere protection protein 1 reduction causes telomere attrition and cellular senescence via Sirtuin 1 deacetylase in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 56(1):38–49. https://doi.org/10.1165/rcmb.2016-0198OC
Chen C, Zhou M, Ge Y, Wang X (2020) SIRT1 and aging related signaling pathways. Mech Ageing Dev 187:111215. https://doi.org/10.1016/j.mad.2020.111215
Barnes PJ (2019) Small airway fibrosis in COPD. Int J Biochem Cell Biol 116:105598. https://doi.org/10.1016/j.biocel.2019.105598
Sarker RSJ, Conlon TM, Morrone C, Srivastava B, Konyalilar N, Verleden SE et al (2019) CARM1 regulates senescence during airway epithelial cell injury in COPD pathogenesis. Am J Physiol Lung Cell Mol Physiol 317(5):L602–L614. https://doi.org/10.1152/ajplung.00441.2018
Li BS, Zhu RZ, Lim SH, Seo JH, Choi BM (2021) Apigenin alleviates oxidative stress-induced cellular senescence via modulation of the SIRT1-NAD+-CD38 Axis. Am J Chin Med 49(5):1235–1250. https://doi.org/10.1142/s0192415x21500592
Hodge G, Tran HB, Reynolds PN, Jersmann H, Hodge S (2020) Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes. Ther Adv Respir Dis 14:1753466620905280. https://doi.org/10.1177/1753466620905280
Kuwano K, Araya J, Hara H, Minagawa S, Takasaka N, Ito S et al (2016) Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). Respir Investig 54(6):397–406. https://doi.org/10.1016/j.resinv.2016.03.010
Diener C, Keller A, Meese E (2022) Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet 38(6):613–626. https://doi.org/10.1016/j.tig.2022.02.006
Munk R, Panda AC, Grammatikakis I, Gorospe M, Abdelmohsen K (2017) Senescence-associated MicroRNAs. Int Rev Cell Mol Biol 334:177–205. https://doi.org/10.1016/bs.ircmb.2017.03.008
Hayek H, Kosmider B, Bahmed K (2021) The role of miRNAs in alveolar epithelial cells in emphysema. Biomed Pharmacother 143:112216. https://doi.org/10.1016/j.biopha.2021.112216
Yao H, Rahman I (2012) Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol 84(10):1332–1339. https://doi.org/10.1016/j.bcp.2012.06.031
Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA et al (2012) SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Investig 122(6):2032–2045. https://doi.org/10.1172/jci60132
Gu C, Zhang Q, Ni D, Xiao QF, Cao LF, Fei CY et al (2020) Therapeutic effects of SRT2104 on lung injury in rats with emphysema via reduction of type II alveolar epithelial cell senescence. COPD 17(4):444–451. https://doi.org/10.1080/15412555.2020.1797657
Di Filippo E, Giampietro L, De Filippis B, Balaha M, Ferrone V, Locatelli M et al (2020) Synthesis and biological evaluation of halogenated-stilbenols as promising antiaging agents. Molecules. https://doi.org/10.3390/molecules25235770
Salla M, Pandya V, Bhullar K, Kerek E, Wong Y, Losch R et al (2020) Resveratrol and resveratrol-aspirin hybrid compounds as potent intestinal anti-inflammatory and anti-tumor drugs. Molecules. https://doi.org/10.3390/molecules25173849
Wang XL, Li T, Li JH, Miao SY, Xiao XZ (2017) The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules. https://doi.org/10.3390/molecules22091529
Navarro S, Reddy R, Lee J, Warburton D, Driscoll B (2017) Inhaled resveratrol treatments slow ageing-related degenerative changes in mouse lung. Thorax 72(5):451–459. https://doi.org/10.1136/thoraxjnl-2016-208964
Wang S, He N, Xing H, Sun Y, Ding J, Liu L (2020) Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1alpha/NF-kappaB signaling axis. J Recept Signal Transduct Res 40(4):388–394. https://doi.org/10.1080/10799893.2020.1738483
Zhang XF, Ding MJ, Cheng C, Zhang Y, Xiang SY, Lu J et al (2020) Andrographolide attenuates oxidative stress injury in cigarette smoke extract exposed macrophages through inhibiting SIRT1/ERK signaling. Int Immunopharmacol 81:106230. https://doi.org/10.1016/j.intimp.2020.106230
Shin NR, Ko JW, Kim JC, Park G, Kim SH, Kim MS et al (2020) Role of melatonin as an SIRT1 enhancer in chronic obstructive pulmonary disease induced by cigarette smoke. J Cell Mol Med 24(1):1151–1156. https://doi.org/10.1111/jcmm.14816
Acknowledgements
The authors would like to thank all the reviewers who participated in the review, MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript, and BioRender (BioRender.com) for the Figure 1.
Funding
This work was supported by the National Natural Science Foundation of China [81500036]; Scientific Research Fund of Hunan Provincial Education Department [21B0005]; Hunan Provincial Natural Science Foundation [2022JJ30917]; Undergraduate Education and Teaching Reform Project of Central South University [2021JGB061]; and Postgraduate Education and Teaching Reform Project of Central South University [2021jy156].
Author information
Authors and Affiliations
Contributions
SL contributed to writing of the original draft preparation. QH contributed to writing, reviewing, and editing of the manuscript. BH contributed to supervision and writing, reviewing, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certified that they had no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Huang, Q. & He, B. SIRT1 as a Potential Therapeutic Target for Chronic Obstructive Pulmonary Disease. Lung 201, 201–215 (2023). https://doi.org/10.1007/s00408-023-00607-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-023-00607-9