Abstract
Purpose
Objective cough frequency is used to assess efficacy of chronic cough (CC) treatments. The objective of this study was to explore the relationship between objective cough frequency and cough-specific patient-reported outcomes (PROs) and estimate a clinically meaningful change threshold (MCT) for objective cough frequency.
Methods
Data collected in a phase 2b study in participants with refractory or unexplained CC were used to investigate the relationship between 24-h cough frequency (measured using an ambulatory cough monitor) and cough-specific PROs (i.e., cough severity visual analog scale, cough severity diary, Leicester Cough Questionnaire). Convergent validity was assessed using Spearman ρ. An MCT for 24-h cough frequency was estimated using the patient global impression of change (PGIC) scale as an anchor.
Results
Correlations between 24-h cough frequency and cough-specific PROs at baseline, Week 4, and Week 12 were significant (P < 0.0001) but low to moderate in strength (ρ = 0.30–0.58). Participants categorized as very much improved/much improved (i.e., PGIC of 1 or 2) or minimally improved (i.e., PGIC of 3) had mean 24-h cough frequency reductions of 55% and 30%, respectively. Receiver operating characteristic curve analysis suggested that a 24-h cough frequency reduction of 38% optimizes sensitivity and specificity for predicting a PGIC score of 1–3.
Conclusion
Objective 24-h cough frequency is significantly associated with cough-specific PROs, but cough frequency and PROs most likely capture distinct aspects of CC. A ≥ 30% reduction in 24-h cough frequency is a reasonable MCT to define treatment response in CC clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic cough (CC) is defined as a cough lasting more than 8 weeks, though some patients with CC experience a cough that occurs daily or almost daily for several years [1,2,3,4,5]. Patients with CC cough frequently, with some patients coughing several dozen times per hour while awake [6,7,8]. Prolonged and frequent coughing can exert substantial burden on patients, inducing negative effects on physical, social, and psychological well-being [3, 4, 9], and cause high healthcare utilization due to repetitive medical visits and treatment trials [3, 10,11,12]. Although treatment of associated medical conditions (e.g., asthma, allergic rhinitis, gastroesophageal reflux disease) can resolve cough, a subset of patients with CC have a cough that persists despite optimal diagnosis and management of associated conditions according to published guidelines [i.e., refractory CC (RCC)] or a cough that persists despite a lack of identifiable, treatable conditions associated with cough after extensive investigation [i.e., unexplained CC (UCC)] [1, 2, 13]. The lack of approved treatments with indications for RCC or UCC reflects a major unmet need.
Monitoring and treating CC is a relatively new field—within the past 2 decades, both objective and subjective tools for assessing cough have been developed [14]. Objective cough monitoring using ambulatory devices to assess reductions in cough after treatment with antitussives has been used in several recent clinical trials in RCC or UCC [6, 7, 15,16,17,18,19], as well as in studies of other respiratory conditions, including acute cough, asthma, and chronic obstructive pulmonary disease (COPD) [20,21,22]. Although cough monitoring is important for evaluating objective efficacy of novel antitussives, this technique is not currently widely available outside of clinical trial settings [14]. As the goal of managing CC in a clinical setting is to improve patients’ symptoms, cough-specific patient-reported outcomes (PROs), such as the cough severity visual analog scale (VAS), cough severity diary (CSD), and Leicester Cough Questionnaire (LCQ), have been developed and validated to monitor various aspects of cough [14, 23]. Because PROs are designed to capture patient-reported aspects of cough (e.g., severity, frequency, intensity, disruption), these subjective tools provide complementary insights into objective cough monitoring when evaluating efficacy of antitussive therapies.
Although both objective cough monitoring and cough-specific PROs have been used in clinical practice and clinical trials, few studies have investigated the relationship between cough frequency and cough-specific PROs [23,24,25]. Clinically meaningful change thresholds (MCTs) for some cough-specific PROs have been established [23, 26, 27], but a within-patient MCT for objective cough frequency has not been estimated. Therefore, we conducted this analysis to evaluate the relationship between objective cough frequency and cough-specific PROs and to estimate an MCT for 24-h cough frequency in patients with RCC or UCC.
Methods
Study Design
For this analysis, we used data from a phase 2b study of the P2X3-receptor antagonist gefapixant (ClinicalTrials.gov identifier: NCT02612610) [6]. Eligible participants had RCC or UCC (per American College of Chest Physicians and British Thoracic Society guidelines) lasting ≥ 1 year and a cough severity VAS ≥ 40 mm. Candidates were excluded if they were current or recent (within 6 months of enrollment) smokers or had substantial chest abnormalities contributing to cough as determined by a chest x-ray within the past 5 years. No eligibility criteria were based on cough frequency. Participants from all treatment groups (i.e., gefapixant and placebo groups) were pooled for this analysis to assess the measurement properties of 24-h cough frequency.
Outcome Measures
Objective cough frequency was measured as previously described [6]. An ambulatory acoustic recording device (VitaloJAK™; Vitalograph Ltd, Buckingham, England) was used to collect sound recordings over 24-h periods, and cough frequencies were assessed by trained analysts after removal of silence and noncough sounds using custom written software. Twenty-four-h cough frequency was calculated as the total number of coughs over the 24-h period divided by 24. There are previous reports with detailed information on the development and validation of the device and software and reliability of the trained analysts [28, 29].
Additional outcomes collected in the phase 2b study included the cough severity VAS, CSD, LCQ, and patient global impression of change (PGIC) scale. The cough severity VAS records patients’ self-assessment of cough severity on a 100-mm linear scale ranging from no cough (0 mm) to worst cough (100 mm) [27]. The CSD is a 7-item questionnaire that assesses the frequency, intensity, and disruptiveness of a patient’s cough on an 11-point scale ranging from 0 to 10, with higher scores indicating greater cough severity; the total CSD score is calculated as the mean of individual item scores [23]. The LCQ assesses cough-specific health-related quality of life (HRQOL) using 19 individual items across physical, social, and psychological domains; each item is measured on a 7-point Likert scale, and the total score is calculated as the sum of individual domain scores (range, 3–21; lower scores reflect worse cough-specific HRQOL) [9]. The PGIC measures overall patient-reported improvement on a 7-point scale ranging from 1 (very much improved) to 7 (very much worse).
Cough frequency, cough severity VAS, and LCQ scores were measured at baseline and Weeks 4, 8, and 12 [6]. The CSD score was calculated weekly as the average of the 7 preceding daily CSD scores and was collected from baseline to Week 12. The PGIC was assessed at Weeks 4, 8, and 12. Participants were categorized into 5 distinct groups based on their PGIC ratings at Weeks 4 and 12: PGIC of 1 or 2 (very much improved or much improved), PGIC of 3 (minimally improved), PGIC of 4 (no change), PGIC of 5 (minimally worse), or PGIC of 6 or 7 (much worse or very much worse).
Statistical Analysis
All statistical tests in this analysis used a significance level of P < 0.05.
Convergent Validity
Convergent validity (or association of related measures) was investigated by comparing 24-h cough frequency with cough severity VAS, CSD, and LCQ at baseline and Weeks 4 and 12. Nonparametric Spearman rank correlation (Spearman ρ) was used for cross-sectional correlations because of the non-normal distribution of cough frequency. Low-to-moderate correlations were expected, as the measures capture similar but conceptually different information.
Known-Groups Validity
To assess known-groups validity of 24-h cough frequency, participants were stratified into severity groups based on the sample distribution at baseline using 2 metrics of severity. The first was based on tertiles of CSD total scores; the second was based on LCQ total score categories of ≤ 8, > 8 and ≤ 13, and > 13. Analysis of variance was used to assess for significant differences in 24-h objective cough frequency between the severity groups, with post hoc category comparisons via the Scheffé test.
Score Interpretation
Anchor- and distribution-based approaches were used to evaluate within-patient MCTs for 24-h cough frequency. Anchor-based estimates of MCTs compared changes in 24-h cough frequency across categories of the 7-point PGIC (1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; 7, very much worse). An MCT was defined as the mean 24-h cough frequency reduction in participants who reported themselves as minimally improved on the PGIC (PGIC of 3). A second anchor-based approach to estimate an MCT was conducted using a receiver operating characteristic (ROC) curve analysis to determine the threshold value for change in 24-h cough frequency from baseline to Week 4, defined as the point on the ROC curve closest to 100% sensitivity and specificity for predicting participants scoring 1–3 on the PGIC (i.e., the ROC point with the shortest difference from the upper left quadrant [0, 1]). Youden index was used to determine the change in 24-h cough frequency that optimized sensitivity and specificity for predicting global improvements on the PGIC.
Distribution-based approaches included one-half of SD and SE of measurement (SEM) at baseline. The former method is calculated as one-half the SD observed at baseline and has been suggested as a good approximation of the minimally important difference [30]. The SEM is calculated by multiplying the baseline SD by the square root of (1 − intraclass correlation coefficient [ICC]), where ICC is test–retest reliability from baseline to Week 4.
Estimates for the within-patient MCT for 24-h cough frequency were made by triangulating results of the anchor- and distribution-based approaches. The agreement between the MCT for 24-h cough frequency estimated in this analysis and the published MCT for the CSD was assessed using the kappa statistic (κ) for agreement between categorical data. This analysis was conducted to compare responder rates defined using a subjective measure (CSD) versus an objective measure (cough frequency). The strength of agreement, reflected by κ, was interpreted using previously published benchmarks [31].
Results
Study Population
Baseline characteristics of participants enrolled in this study were previously published [6, 32]. Mean (SD) baseline scores were 29.5 (39.4) coughs/h for 24-h cough frequency, 57.5 (22.3) mm for cough severity VAS, 4.3 (1.9) points for CSD total score, and 11.7 (3.0) points for LCQ total score.
Convergent Validity
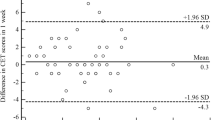
Correlations were assessed between 24-h cough frequency and cough-specific PRO total and domain scores at baseline and Weeks 4 and 12 using Spearman ρ (Table 1; Fig. 1). Correlations were low to moderate at each cross-sectional time point.
Scatter plots (with outliers removed) of 24-h cough frequency versus a cough severity VAS, b mean weekly CSD total scores, and c LCQ total scores. One outlier data point has been removed from each plot to aid visualization. CSD, cough severity diary; LCQ, Leicester Cough Questionnaire; VAS, visual analog scale
Known-Groups Validity
In support of known-groups validity, baseline mean 24-h cough frequencies increased with increases in CSD total scores (F = 7.70; P = 0.0006; Table 2). Additionally, mean 24-h objective cough frequencies were lowest in participants with the highest LCQ total scores (indicating better cough-specific HRQOL) and increased with decreasing LCQ total scores (F = 15.56; P < 0.001).
Score Interpretation
Anchor-based estimates of within-patient MCTs were conducted using PGIC category as an anchor. There was a trend toward greater reductions in 24-h cough frequency for groups reflecting greater improvements according to the PGIC (Table 3). Participants who reported themselves as minimally improved (PGIC of 3) at Weeks 4 and 12 had a reduction in 24-h cough frequency of approximately 30%, reflecting a much greater change than the approximately 2–6% reductions observed in the no change PGIC group (PGIC of 4) over the same time periods. Participants with greatest improvements according to the PGIC (PGIC of 1 or 2) had the highest reductions in 24-h cough frequency (Week 4, 58%; Week 12, 55%). Similar results were observed when examining trends in awake cough frequency reductions across PGIC categories (Online Resource 1).
An ROC analysis was also conducted at Week 4 using a PGIC score of 1–3 as an anchor (Fig. 2). Sensitivity and specificity in predicting a PGIC score of 1–3, as measured by the Youden index, was optimized at a 38% reduction in 24-h cough frequency (Table 4).
Mean (SD) 24-h cough frequency at baseline was 29.5 (39.4) coughs/h. Estimates for the distribution-based MCT were 19.7 (one-half of SD) and 15.9 (SEM) coughs/h.
A ≥ 30% reduction in 24-h cough frequency, based on triangulating threshold estimates from anchor-based analyses, was identified as a potential MCT. The proportion of participants who met this responder definition for 24-h cough frequency was compared with the proportion of participants who were responders according to a previously estimated MCT for the CSD (i.e., ≥ 1.3-point reduction in total CSD score [23]). Among 125 participants who were responders by the 24-h cough frequency responder definition at Week 4, 83 (66%) were also responders on the CSD. Among 87 participants who were nonresponders by the 24-h cough frequency responder definition at Week 4, 57 (65%) were also nonresponders on the CSD. The kappa statistic for agreement between measures was consistent, with fair agreement between the 24-h cough frequency and CSD responder definitions (κ = 0.31; P < 0.0001). Similar agreement was observed for the ≥ 30% reduction in 24-h cough frequency responder definition with both the cough severity VAS (≥ 30-mm reduction) and LCQ (≥ 1.3-point increase) responder definitions at Week 4. Among participants with cough severity VAS data, 57% (72/127) of cough frequency responders were cough severity VAS responders and 81% (75/93) of cough frequency nonresponders were cough severity VAS nonresponders. Among participants with LCQ data, 81% (102/126) of cough frequency responders were LCQ responders and 57% (58/102) of cough frequency nonresponders were LCQ nonresponders.
Discussion
To our knowledge, this is the first analysis formally estimating an MCT for 24-h cough frequency in patients with RCC or UCC. This analysis demonstrates that 24-h cough frequency is moderately but significantly correlated with cough-specific PROs and estimates a ≥ 30% reduction as an MCT for 24-h cough frequency.
Objective cough monitoring and cough-specific PROs capture distinct yet complementary aspects of cough, with PROs capturing subjective evaluations of cough, which can vary greatly among patients (even among those with similar cough frequencies depending on the perceived effect of cough on their daily lives). Consistent with this consideration, the correlation coefficients between 24-h cough frequency and cough-specific PROs observed in the current study were low to moderate in strength when analyzed at a single time point and were similar to previously reported correlations between cough frequency and cough-specific PROs [24, 33, 34]. Additionally, relationships between cough frequency and cough severity VAS and cough frequency and LCQ in the current analysis strengthened over time, suggesting that improvements in cough frequency may be closely correlated to improvements in other aspects of cough (e.g., cough-specific HRQOL) than comparisons at single time points. Ultimately, this study reinforces that objective and subjective measures of cough are complementary and, when taken together, describe the multifaceted impact of cough on patients.
An anchor-based approach using a participant-reported minimal change on the PGIC (PGIC of 3) suggested an approximate MCT of a 30% reduction, whereas participants reporting themselves as very much improved or much improved (PGIC of 1 or 2) had reductions in 24-h cough frequency of approximately 50%. The ROC curve–based analysis (which categorized a response as a PGIC score of 1–3) suggested a change threshold for 24-h cough frequency between these 2 estimates (i.e., an approximate 40% reduction). Meanwhile, distribution-based estimates produced a more robust MCT (i.e., 16–20 coughs/h, or an approximate 50–70% reduction given the baseline mean 24-h cough frequency of 30 coughs/h) due to the large variability in cough frequency observed in the patient population. Notably, an MCT expressed as a percentage rather than absolute reduction in cough frequency was considered more meaningful given that percentage reductions are a standard measure for reporting in CC clinical trials, inherently adjust for variation in baseline cough frequency, and are more interpretable from a clinical perspective. An MCT based on absolute change would have poor generalizability to those with higher or lower baseline cough frequencies.
Overall, these data suggest a minimum MCT of 30% as an appropriate target when assessing cough frequency reduction after antitussive therapy, though use of more stringent thresholds may also be reasonable. In primary findings from the clinical trial used as the source for the current analysis, 80% of participants treated with gefapixant 50 mg were responders by the ≥ 30% reduction threshold (vs. 44% of participants who received placebo) [6]. Moreover, 51% of participants treated with gefapixant 50 mg had a ≥ 50% reduction in awake cough frequency (vs. 25% in the placebo group). These data suggest reduction thresholds of 30% and 50% are achievable outcomes in clinical trials assessing novel treatments of RCC or UCC. Further studies investigating whether the 30% cough frequency reduction threshold can discriminate between effective and noneffective interventions for RCC or UCC are warranted.
There are limitations to the current study. First, all enrolled participants were from the USA or UK and had moderate to severe (i.e., baseline cough severity VAS ≥ 40 mm) and long-lasting CC. Therefore, confirming these findings in a broader geographic patient population and among patients with less severe CC or more recent-onset CC is warranted. Second, as this trial enrolled participants with RCC or UCC, the MCT determined in this analysis may not directly apply to patients with other causes of CC (e.g., COPD). Finally, the timeline for assessing meaningful change was extended through only 12 weeks of treatment, and analyses using longer treatment periods may be warranted to examine the stability and consistency of these results.
In conclusion, this analysis provides insights into the relationship between objective cough frequency and subjective cough-specific PROs for monitoring CC and provides MCT estimates for use in CC clinical trials or clinical practice.
References
Morice AH, Millqvist E, Bieksiene K et al (2020) ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 55(1):1901136
Irwin RS, French CL, Chang AB et al (2018) Classification of cough as a symptom in adults and management algorithms: CHEST Guideline and Expert Panel Report. Chest 153(1):196–209
Chamberlain SA, Garrod R, Douiri A et al (2015) The impact of chronic cough: a cross-sectional European survey. Lung 193(3):401–408
Koskela HO, Lätti AM, Purokivi MK (2017) Long-term prognosis of chronic cough: a prospective, observational cohort study. BMC Pulm Med 17(1):146
Yousaf N, Montinero W, Birring SS et al (2013) The long term outcome of patients with unexplained chronic cough. Respir Med 107(3):408–412
Smith JA, Kitt MM, Morice AH et al (2020) Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med 8(8):775–785
Smith JA, Kitt MM, Butera P et al (2020) Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J 55(3):1901615
Kelsall A, Decalmer S, McGuinness K et al (2009) Sex differences and predictors of objective cough frequency in chronic cough. Thorax 64(5):393–398
Birring SS, Prudon B, Carr AJ et al (2003) Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 58(4):339–343
Koskela HO, Lätti AM, Pekkanen J (2019) Risk factors for repetitive doctor’s consultations due to cough: a cross-sectional study in a Finnish employed population. BMJ Open 9(6):e030945
Holden SE, Morice A, Birring SS et al (2020) Cough presentation in primary care and the identification of chronic cough: a need for diagnostic clarity? Curr Med Res Opin 36(1):139–150
Zeiger RS, Schatz M, Butler RK et al (2020) Burden of specialist-diagnosed chronic cough in adults. J Allergy Clin Immunol Pract 8(5):1645-1657.e7
McGarvey L, Gibson PG (2019) What is chronic cough? Terminology. J Allergy Clin Immunol Pract 7(6):1711–1714
Cho PSP, Birring SS, Fletcher HV et al (2019) Methods of cough assessment. J Allergy Clin Immunol Pract 7(6):1715–1723
Belvisi MG, Birrell MA, Wortley MA et al (2017) XEN-D0501, a novel transient receptor potential vanilloid 1 antagonist, does not reduce cough in patients with refractory cough. Am J Respir Crit Care Med 196(10):1255–1263
Birring SS, Wijsenbeek MS, Agrawal S et al (2017) A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med 5(10):806–815
Khalid S, Murdoch R, Newlands A et al (2014) Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol 134(1):56–62
Smith J, Allman D, Badri H et al (2020) The neurokinin-1 receptor antagonist orvepitant is a novel antitussive therapy for chronic refractory cough: results from a phase 2 pilot study (VOLCANO-1). Chest 157(1):111–118
Smith JA, McGarvey LPA, Badri H et al (2017) Effects of a novel sodium channel blocker, GSK2339345, in patients with refractory chronic cough. Int J Clin Pharmacol Ther 55(9):712–719
Marsden PA, Satia I, Ibrahim B et al (2016) Objective cough frequency, airway inflammation, and disease control in asthma. Chest 149(6):1460–1466
Sumner H, Woodcock A, Kolsum U et al (2013) Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 187(9):943–949
Sunger K, Powley W, Kelsall A et al (2013) Objective measurement of cough in otherwise healthy volunteers with acute cough. Eur Respir J 41(2):277–284
Martin Nguyen A, Bacci E, Dicpinigaitis P et al (2020) Quantitative measurement properties and score interpretation of the Cough Severity Diary in patients with chronic cough. Ther Adv Respir Dis 14:1753466620915155
Kelsall A, Decalmer S, Webster D et al (2008) How to quantify coughing: correlations with quality of life in chronic cough. Eur Respir J 32(1):175–179
Kelsall A, Houghton LA, Jones H et al (2011) A novel approach to studying the relationship between subjective and objective measures of cough. Chest 139(3):569–575
Raj AA, Pavord DI, Birring SS (2009) Clinical cough IV: What is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol 187:311–320
Martin Nguyen A, Bacci ED, Vernon M et al (2021) Validation of a visual analog scale for assessing cough severity in patients with chronic cough. Ther Adv Respir Dis 15:17534666211049744
Mines D, Bacci E, Nguyen AM et al (2019) Assessment of inter- and intra-rater reliability of objective cough frequency in patients with chronic cough (abstract). Eur Respir J 54:PA4342
Smith JA, Holt K, Dockry R et al (2021) Performance of a digital signal processing algorithm for the accurate quantification of cough frequency. Eur Respir J 58(2):2004271
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41(5):582–592
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Morice AH, Birring SS, Smith JA et al (2021) Characterization of patients with refractory or unexplained chronic cough participating in a phase 2 clinical trial of the P2X3-receptor antagonist gefapixant. Lung 199(2):121–129
Fletcher H, Cho PSP, Matos S et al (2017) Chronic cough: objective cough frequency in a large cough clinic cohort (abstract). Eur Respir J 50(Suppl 61):OA4677
Faruqi S, Thompson R, Wright C et al (2011) Quantifying chronic cough: objective versus subjective measurements. Respirology 16(2):314–320
Acknowledgements
Medical writing and editorial assistance were provided under the direction of the authors by Nathan Rodeberg, PhD, and Jenna Lewis, MA, ELS, of MedThink SciCom. This assistance was funded by Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
SSB, MV, CL, and JAS contributed to study conception and design. SSB and JAS contributed to acquisition of data. JS, AMN, EDB, and MV contributed to data analysis. All authors contributed to interpretation of the data, critically revised the manuscript for important intellectual content, approved of the final version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
JS, AMN, DRM, and CL are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may hold stock or stock options in Merck & Co., Inc., Rahway, NJ, USA. SSB reports grants from Merck & Co., Inc.; personal fees for advisory board work from Bayer, Bellus, GSK, Menlo, Merck & Co., Inc., Nocion, Sanofi, and Shionogi; and reimbursement for travel expenses from Boehringer Ingelheim. EDB is an employee of Evidera, which provides consulting and other research services to pharmaceutical, medical device, and related organizations. In her salaried position, she works with a variety of companies and organizations and is precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to participate in the study and the development of this manuscript. MV reports nonfinancial support from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and personal fees from Evidera during the conduct of the study. JAS reports grants and personal fees related to the submitted work from Afferent Pharmaceuticals/Merck & Co., Inc.; grants from Ario Pharma, Bayer, Bellus, GlaxoSmithKline, Menlo, and NeRRe Pharmaceuticals; personal fees from Ario Pharma, Bayer, Bellus, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Menlo, Neomed, and NeRRe Pharmaceuticals; nonfinancial support from Vitalograph; and is a named inventor on a patent, owned by Manchester University NHS Foundation Trust and licensed to Vitalograph Ltd, describing the detection of cough from sound recordings.
Ethical Approval
The phase 2b study (ClinicalTrials.gov, NCT02612610) was approved by the Investigational Review Boards or Ethics Review Committees of the 44 study centers in the UK and USA, and done in accordance with the principles of Good Clinical Practice.
Consent to Participate
Patients gave written informed consent before enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schelfhout, J., Nguyen, A.M., Birring, S.S. et al. Validation and Meaningful Change Thresholds for an Objective Cough Frequency Measurement in Chronic Cough. Lung 200, 717–724 (2022). https://doi.org/10.1007/s00408-022-00587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00587-2